Domingo F. Barber
Group Leader
Research summary
The overall objective of our group is to understand nanomedicine-mediated molecular and cellular mechanisms in new nanomedicine-based therapeutic applications developed for the treatment of cancer or autoimmunity, and to use this knowledge to bring these therapies closer to clinical application.
Publications
Egea-Benavente D, Ovejero JG, Morales MDP, Barber DF. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers (Basel). 2021 Sep 12;13(18):4583.
Mulens-Arias V, Rojas JM, Barber DF. The Use of Iron Oxide Nanoparticles to Reprogram Macrophage Responses and the Immunological Tumor Microenvironment. Front Immunol. 2021 Jun 9;12:693709.
Portilla Y, Mellid S, Paradela A, Ramos-Fernández A, Daviu N, Sanz-Ortega L, Pérez-Yagüe S, Morales MP, Barber DF. Iron Oxide Nanoparticle Coatings Dictate Cell Outcomes Despite the Influence of Protein Coronas. ACS Appl Mater Interfaces. 2021 Feb 24;13(7):7924-7944.
Sanz-Ortega L, Rojas JM, Barber DF. Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting. Pharmaceutics. 2020 Aug 27;12(9):812.
Mulens-Arias V, Rojas JM, Barber DF. The Intrinsic Biological Identities of Iron Oxide Nanoparticles and Their Coatings: Unexplored Territory for Combinatorial Therapies. Nanomaterials (Basel). 2020 Apr 27;10(5):837.
Mulens-Arias V, Rojas JM, Sanz-Ortega L, Portilla Y, Pérez-Yagüe S, Barber DF Polyethylenimine-coated superparamagnetic iron oxide nanoparticles impair in vitro and in vivo angiogenesis. Nanomedicine 2019;Oct;21:102063. doi: 10.1016/j.nano.2019.102063.
Magnetic Iron oxide nanoparticles (MNPs) have considerable potential to be used as nanomedicines for targeted drug release or magnetic resonance imaging. More recently, we highlighted the promise of using MNPs in other therapeutic approaches to treat cancer, such as the induction of intracellular hyperthermia in tumour cells or the magnetic targeting/retention of lymphocytes in cell transfer therapies. We have also seen that the accumulation of MNPs by different cell types induces oxidative stress and its associated effects as a consequence of MNP degradation. Thus, here we aim to explore whether these responses could be used therapeutically to fight tumours at different levels.
The overall objective of our group is to fully understand the molecular and cellular mechanisms induced by MNPs at their different levels of action. This knowledge can be used to improve the functional design of MNPs for specific biomedical applications, such as therapies to combat tumours and autoimmune diseases, with the aim of bringing them closer to their clinical application.
As such, we will pursue five specific objectives:
1) We will expand our studies on the magnetic retention/accumulation of MNP-functionalized anti-tumour lymphoid cells in ACT therapies in order to bring this therapy closer to the clinic;
2) We intend to explore whether the targeting to and/or retention of MNP loaded tolDCs in LNs could ameliorate the symptoms of lupus in the MRL/lpr mouse model of SLE;
3) We will evaluate the capacity of the oxidative stress induced in cells by MNPs to remodel the tumour microenvironment and to improve anti-cancer therapies;
4) We will assess how to improve the efficiency of intracellular heating of MNPs in AMF-induced hyperthermia strategies, studying the biological effects induced by MNPs of different physico-chemical characteristics (size, shape, anisotropy) after the application of an AMF of different intensity and frequency;
5) We will analyse whether oxidative and endoplasmic reticulum (ER) stress caused by MNPs inside tumour cells could affect the processing and presentation of antigens, and whether this might provoke the generation of neoantigens.
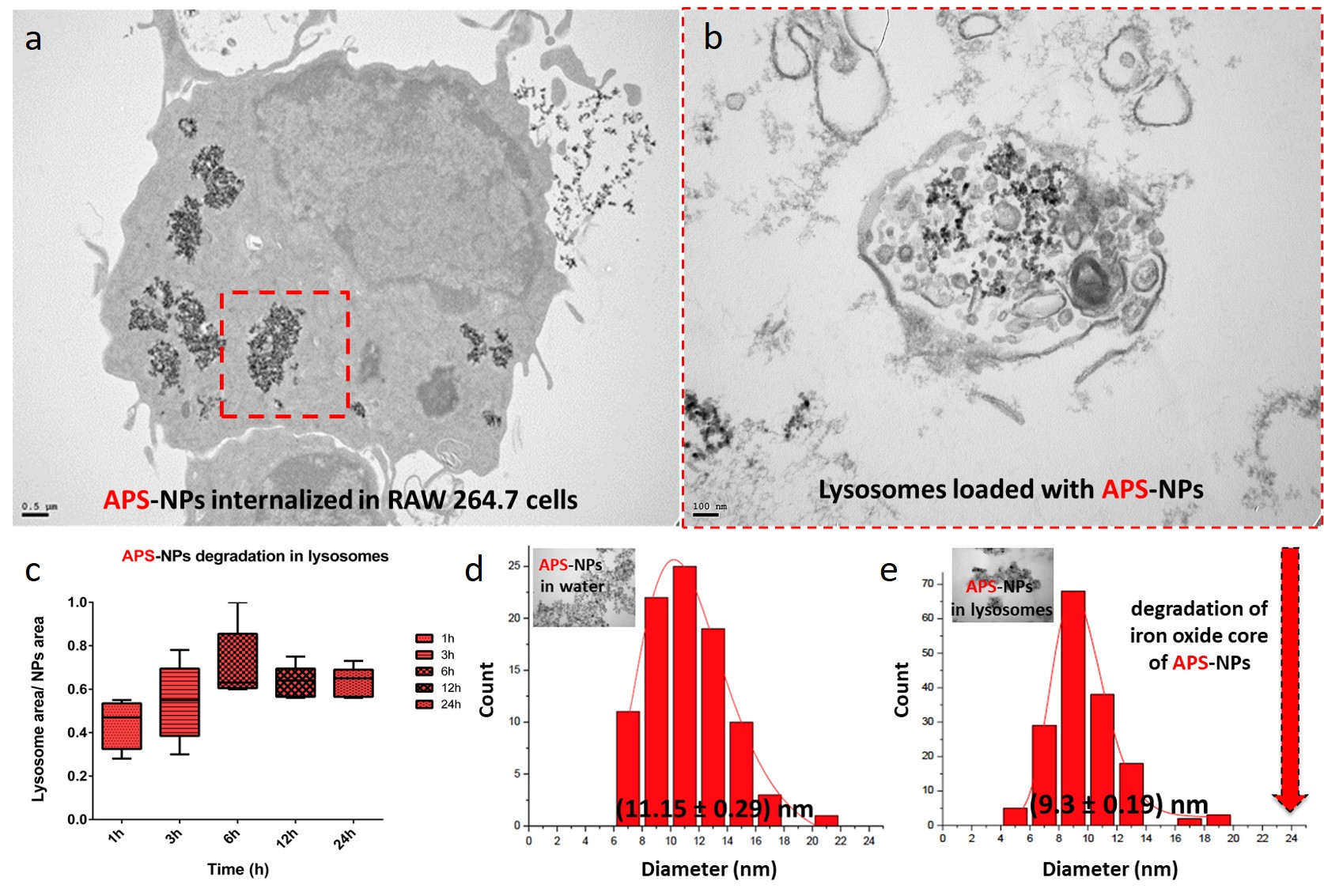
Figure legend. Lysosomal degradation of superparamagnetic iron oxide nanoparticles inside of macrophages. (a) TEM images of typical subcellular localizations of APS-NPs. RAW 264.7 cells were exposed to 125 μg/mL APS-coated iron oxide nanoparticles in DMEM for 24 hours. After 24 hours most of the observed particles were found inside lysosomes. (b) TEM images of the organellar fractions isolated by magnetic extraction from cells treated for 24 h with APS-NPs confirmed presence of nanoparticles in the isolated organelles. (c) Size reduction of APS-NPs during the degradation process inside the lysosomes over 24h. Transmission electron micrographs of the APS-NPs at the initial (d) and final (e) points of the degradation process together with the particle core size distribution measurements obtained from the TEM images. Scale bar: 100 nm. (Yadileiny Portilla).
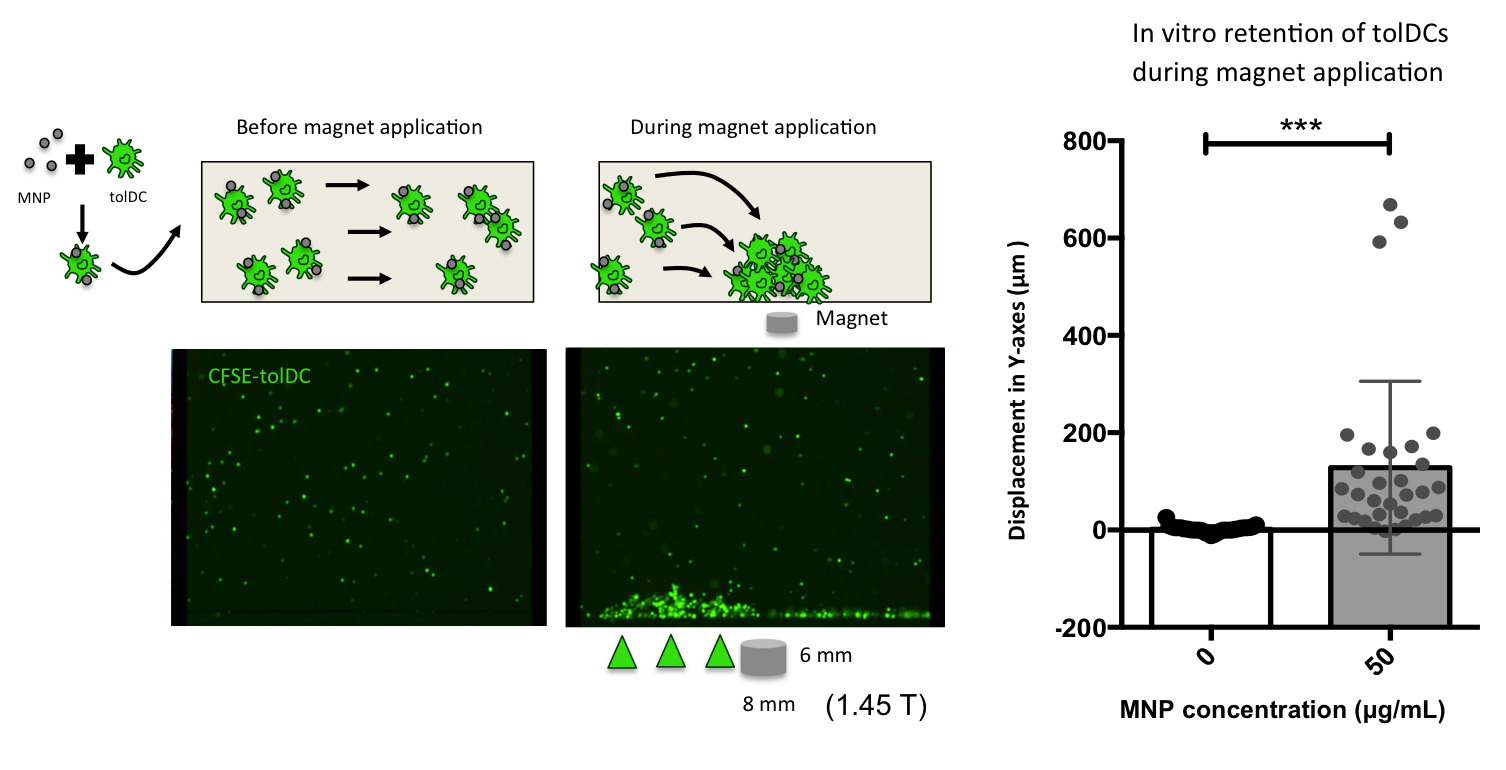
Figure legend. Retention of tolerogenic dendritic cells (tolDCs) associated to Magnetic nanoparticles (MNPs) using a neodymium magnet of 1.45 T (8 x 6 mm) in a flow chamber assay. Fluorescent microscopy videos were used to quantify the displacement in the magnetic force direction (Y-axes). (Andrés París).








