Carlos Ardavín
Group Leader
Research summary
Carlos Ardavín´s lab is focused on the immunobiology of monocytes, macrophages and dendritic cells during airway allergy and bacterial and fungal infections, and over the last years on the crosstalk between inflammation and coagulation in the control of innate immunity in the peritoneal cavity, using murine experimental models of bacterial abdominal sepsis and peritoneal metastasis.
Publications
Feo-Lucas L, Godio C, Minguito de la Escalera M, Alvarez-Ladrón N, H. Villarrubia L, Vega-Pérez A, González-Cintado L, Domínguez-Andrés J, García-Fojeda B, Montero-Fernández C, Casals C, Chiara Autilio C, Pérez-Gil J, Crainiciuc G, Hidalgo A, López-Bravo M, Ardavín C. Airway allergy causes alveolar macrophage death, profound alveolar disorganization and surfactant dysfunction. Frontiers in Immunology 2023; 14:1125984
Ferriz M, Vega-Pérez A, Gutiérrez-González A, Alvarez-Ladrón N, Ardavín C. Whole mount immunofluorescence imaging and isolation of mesothelium-bound immune cell aggregates during mouse peritoneal inflammation. STAR Protocols 2023, 4:102079
Ardavín C, Álvarez-Ladrón N, Ferriz M, Gutiérrez-González A, Vega-Pérez A. Mouse tissue-resident peritoneal macrophages in homeostasis, repair, infection and tumor metastasis. Advanced Science 2023, e2206617
Vega-Pérez A, Villarrubia LH, Godio C, Gutiérrez-González A, Feo-Lucas L, Ferriz M, Martínez-Puente N, Alcaín J, Mora A, Sabio G, López-Bravo M, Ardavín C. Resident macrophage-dependent immune cell scaffolds drive anti-bacterial defense in the peritoneal cavity. Immunity 2021; Oct 25;S1074-7613(21)00444-1.
Domínguez-Andrés J, Feo-Lucas L, Minguito de la Escalera M, González L, López-Bravo M, Ardavín C. Inflammatory Ly6Chigh monocytes protect against candidiasis through IL-15-driven NK cell/neutrophil activation. Immunity 2017; 46: 1059-107
1- Innate immunity against systemic fungal infection
Neutrophils are known to play a crucial role in defense against systemic candidiasis, a disease associated with a high mortality rate in patients receiving immunosuppressive therapy; however the early immune mechanisms that boost the candidacidal activity of neutrophils remain to be defined in-depth.
Using a murine model of systemic candidiasis to explore the role of inflammatory monocytes in NK cell-mediated neutrophil activation in defense against Candida albicans, we found that efficient anti-Candida immunity requires a collaborative response between the spleen and kidney, which relies on type I interferon-dependent IL-15 production by spleen inflammatory monocytes to drive efficient activation and GM-CSF release by spleen NK cells. This in turn is necessary to boost the Candida killing potential of kidney neutrophils(Domínguez-Andrés et al., Immunity 2017). These findings unveil a novel role for IL-15 as a critical mediator in defense against systemic candidiasis, and hold promise for the design of IL-15-based antifungal immunotherapies.
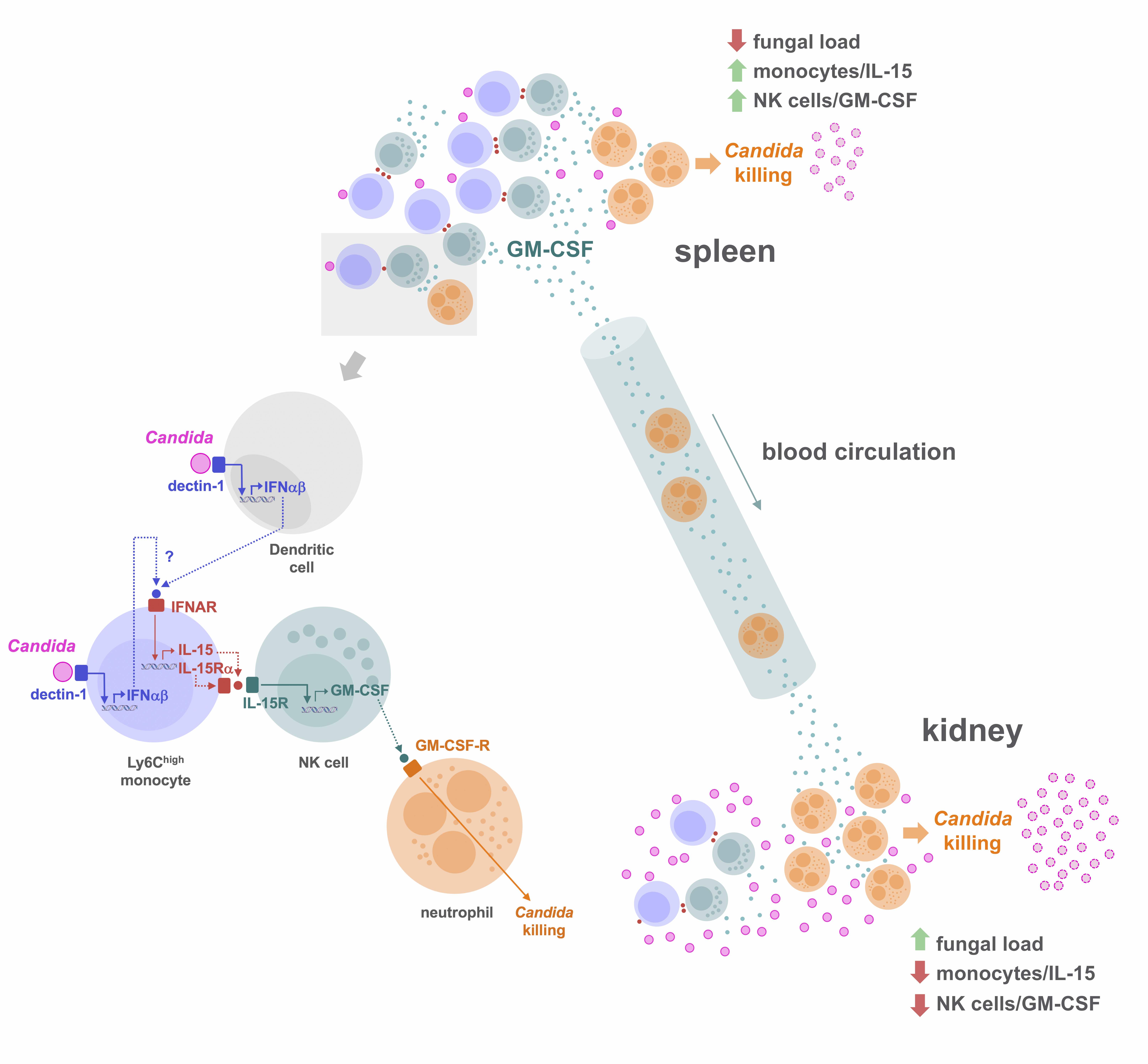
2- Inflammation-coagulation crosstalk in peritoneal innate immunity
Abdominal sepsis
The mechanisms linking the exacerbated inflammation caused by intra-abdominal infection to tissue damage, coagulopathy and organ dysfunction during sepsis have been extensively investigated. However, the defense mechanisms responsible for the control of intraperitoneal bacterial infection in non-lethal sepsis, remain poorly understood.
With the aim of exploring the response of peritoneal immune cells to bacterial challenge, and to understand how peritoneal immune cells, free in the peritoneal fluid in homeostasis, interact and fulfill their defense functions in the absence of an extracellular stromal support, we used an intra-abdominal sepsis model based on the intraperitoneal administration of a sublethal inoculum of E. coli, a prevalent bacterial species in sepsis caused by Gram-negative bacteria.
Studies carried out in Carlos Ardavín lab demonstrate that large peritoneal macrophages (LPMs), classically linked to peritoneal homeostasis, are crucial for defense against E. coli infection by coordinating the formation of mesothelium-bound, multicellular structures, composed by sequentially-recruited LPMs, B1-cells, neutrophils and monocyte-derived cells (moCs), that we termed resMØ-aggregates.
resMØ-aggregates are dynamic, transient structures, that provide a physical scaffold allowing the interaction and function of peritoneal immune cells and enable efficient control of infection. The formation of resMØ-aggregates requires the development of a fibrin scafold resulting from mesothelial tissue factor-dependent fibrin polymerization, triggered by LPM-mesothelial interactions (Vega-Pérez et al., Immunity 2021).
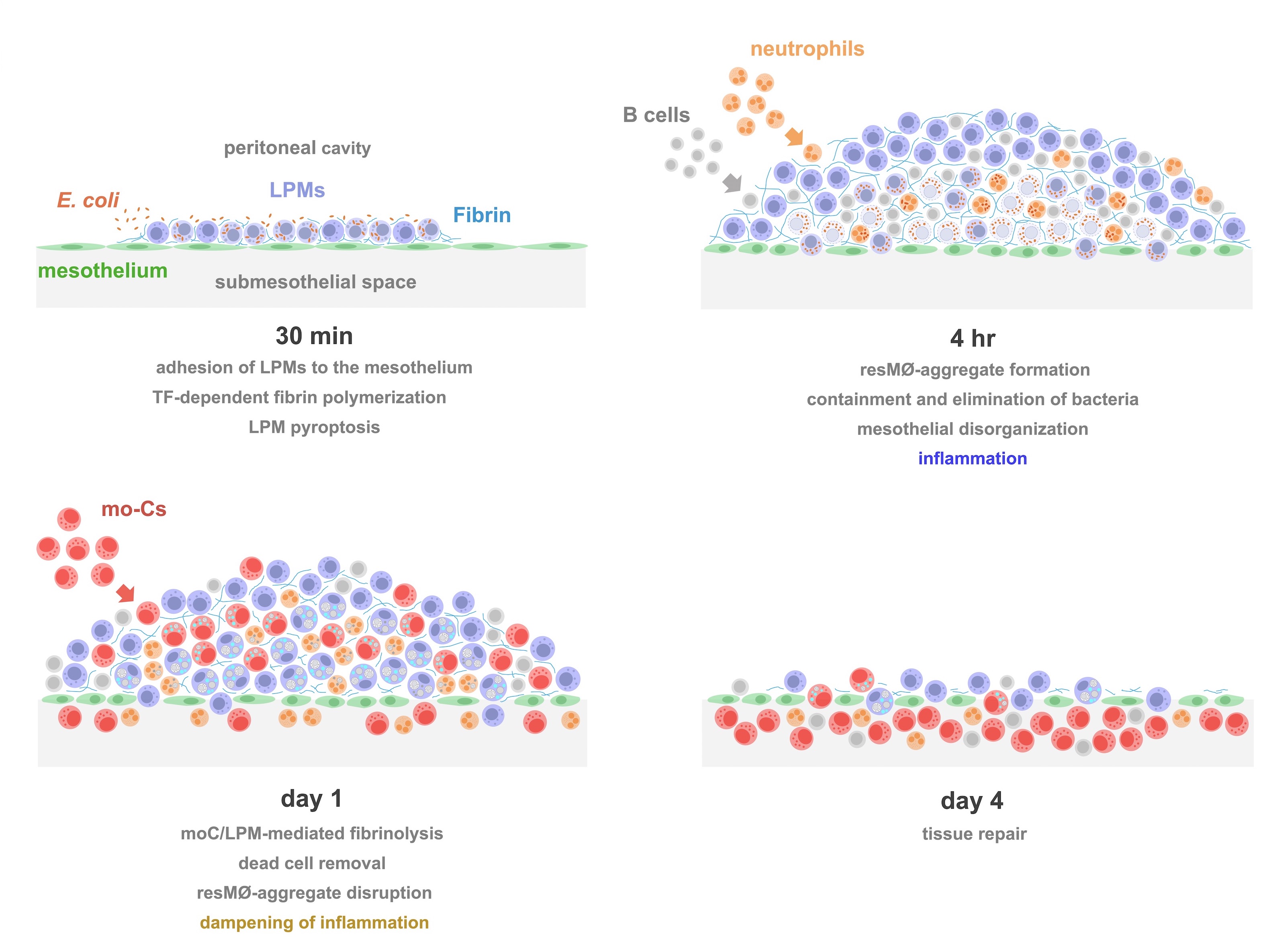
Integrated model of the formation, evolution and disruption of resMØ-aggregates induced by intraperitoneal E. coli infection
Colorectal tumor peritoneal metastasis
Colorectal cancer is the third most common cancer and the fourth most common cause of cancer-associated death worldwide, being the metastatic disease the principal cause of mortality. Besides the lymphatic and hematogenous routes of metastasis, colorectal cancer gives rise to tumor cell dissemination in the peritoneal cavity, which leads to peritoneal tumor metastasis, which develop in 5-15% of colorectal cancer patients. An in-depth understanding of the mechanisms controlling the response of the peritoneal immune system to peritoneal tumor metastasis is needed to develop immunotherapeutical treatments as an alternative to the current therapeutical stategy based on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, associated to severe adverse effects.
The experimental model developed in Carlos Ardavin lab to explore the response of the peritoneal innate immune system to intraperitoneal colorectal cancer metastasis relies on the intraperitoneal or intracecal transfer of mouse tumor organoids, derived from primary tumors or liver mnetastasis. Ongoing research in this area involves monitoring of metastatic growth in the peritoneal wall and omentum in control and genetically-modified mouse lines, by IVIS and whole mount immunofluorescence combined with confocal microscopy, kinetics and transcriptomic analyses of resident and recruited peritoneal cells, with the aim of exploring the interplay between peritoneal immune cells, mesothelial cells and coagulation driving peritoneal metastatic growth.
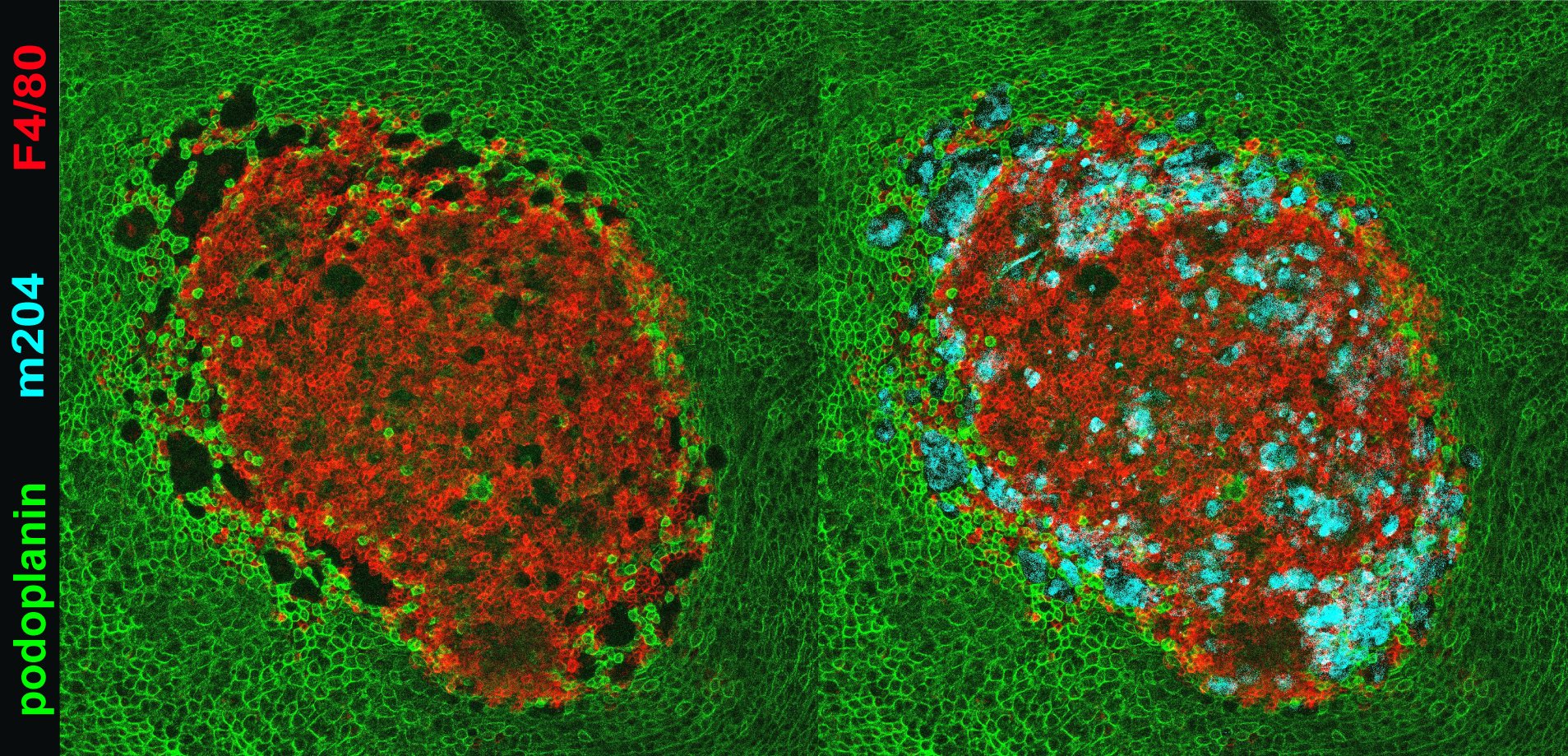
Whole mount immunofluorescence and confocal microscopy image of a colorectal tumor metastasis in the omentum 24 hr after intraperitoneal injection of tumor organoids. F4/80: resident macrophages; m204 tumor cells; podoplanin: mesothelium








