Luis Enjuanes
Group Leader
Isabel Sola
Group Co-leader
Research summary
The main focus of our research is the study of the molecular basis of coronavirus (CoV) replication and virulence, and the identification of signalling pathways modified by the virus, to control disease. The information from these basic projects will be used to design protection strategies against CoV-induced diseases, particularly human severe pneumonia that can end in acute respiratory distress syndrome (ARDS).
Publications
Morales L, Oliveros JC, Fernandez-Delgado R, tenOever BR, Enjuanes L, Sola I. SARS-CoV-Encoded Small RNAs Contribute to Infection-Associated Lung Pathology. Cell Host Microbe. 2017;21(3):344-355
Canton J, Fehr AR, Fernandez-Delgado R, Gutierrez-Alvarez FJ, Sanchez-Aparicio MT, Garcia-Sastre A, Perlman S, Enjuanes L, Sola I.MERS-CoV 4b protein interferes with the NF-kappaB-dependent innate immune response during infection. PLoS Pathog 2018; 14: e1006838.
Castaño-Rodriguez C, Honrubia JM, Gutierrez-Alvarez J, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeno JM, Regla-Nava JA, Fernandez-Delgado R, Verdia-Baguena C, Queralt-Martin M, Kochan G, Perlman S, Aguilella V, Sola I, Enjuanes, LRole of severe acute respiratory syndrome coronavirus viroporins E, 3a and 8a in replication and pathogenesis. mBio 2018 9: e02325-17
Stalin Raj V, Okba NMA, Gutierrez-Alvarez J, Drabek D, van Dieren B, Widagdo W, Lamers MM, Widjaja I, Fernandez-Delgado R, Sola I, Bensaid A, Koopmans MP, Segales J, Osterhaus A, Bosch BJ, Enjuanes L, Haagmans BLChimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. . Sci Adv 2018; 4: eaas9667
Letko M, Miazgowicz K, McMinn R, Seifert SN, Sola I, Enjuanes L, Carmody A, van Doremalen N, Munster V Adaptive evolution of MERS-CoV to species variation in DPP4.. Cell Rep 2018; 24: 1730-1737.
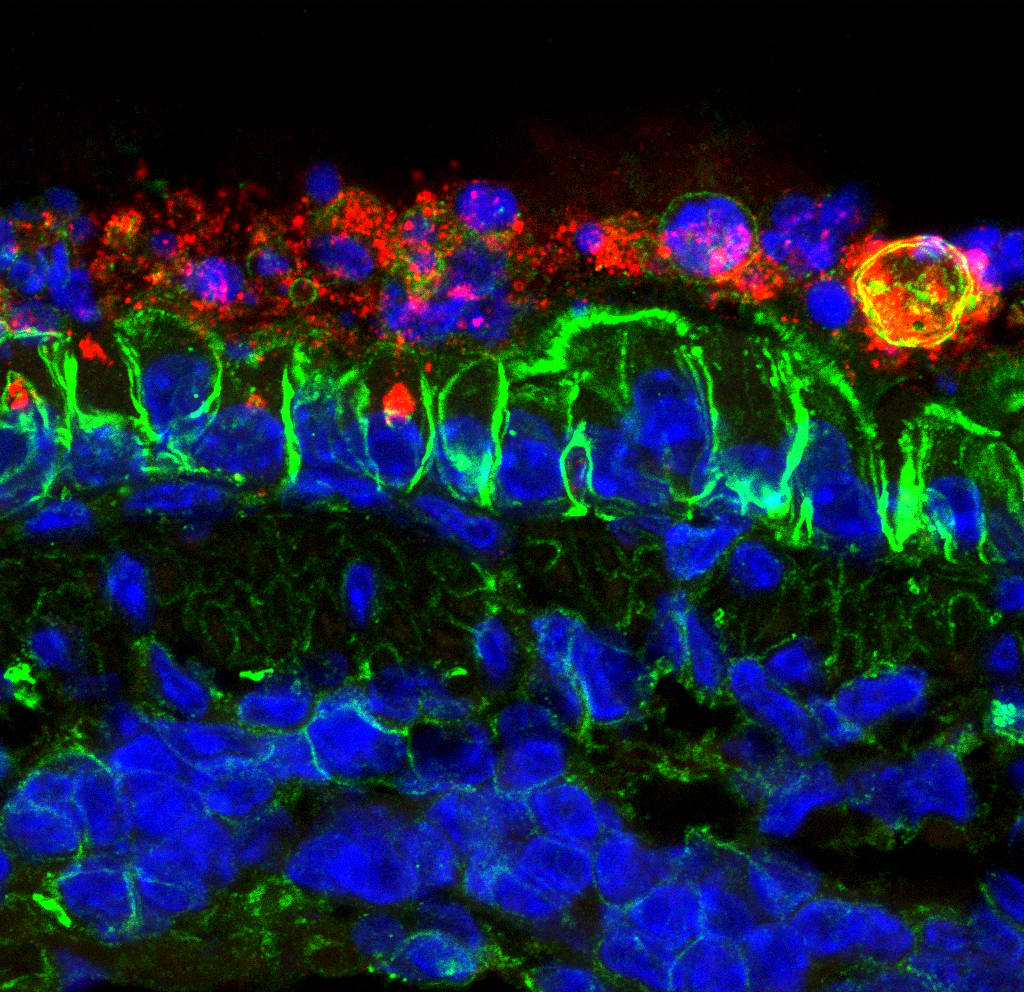 Human infections causing pneumonia and acute respiratory distress syndrome (ARDS) are a growing health problem. In 2015, respiratory diseases were the third most common cause of death in the EU. The problem is even greater in the elderly population, which responds with significant lower efficacy to vaccination. Viruses are responsible for most respiratory infections. Among them, human coronaviruses (CoV) are the cause of up to 15% of all respiratory problems. Seven human CoVs have been described, HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV, MERS-CoV, and 2019-nCoV, the last three leading to deadly infections. Our laboratory focuses on the design of vaccines and selection of antivirals to protect against human respiratory CoV infections by modulating the innate immune response in young and elderly populations.
Human infections causing pneumonia and acute respiratory distress syndrome (ARDS) are a growing health problem. In 2015, respiratory diseases were the third most common cause of death in the EU. The problem is even greater in the elderly population, which responds with significant lower efficacy to vaccination. Viruses are responsible for most respiratory infections. Among them, human coronaviruses (CoV) are the cause of up to 15% of all respiratory problems. Seven human CoVs have been described, HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV, MERS-CoV, and 2019-nCoV, the last three leading to deadly infections. Our laboratory focuses on the design of vaccines and selection of antivirals to protect against human respiratory CoV infections by modulating the innate immune response in young and elderly populations.
The main aims of our research are:
• To identify CoV genes responsible for virus virulence, to delete or modify these genes using reverse genetics in order to develop new generation vaccines such as replication-competent propagation-deficient RNA replicons, which are safe and promising vaccine candidates, and to determine their effectiveness in animal model systems. The expression of micro-RNAs with immunomodulating capacities will provide enhanced efficiency in older adults.
• To identify cell-signalling pathways involved in CoV replication and pathology, and to select antiviral drugs that inhibit these pathways interfering with virus replication or pathology. In particular, we study PBM-PDZ protein-protein interactions involved in the innate immune and inflammatory responses, since overstimulation of these pathways seems responsible for an increase in fatalities during SARS-CoV, MERS-CoV and 2019-nCoV epidemics
• To determine the contribution of host miRNAs and virus-derived small RNAs to the inflammatory lung pathology induced by CoV infection. These small non-coding RNAs represent targets for antivirals
• To study the effect of specific IFN-stimulated genes on the replication and innate immune responses of respiratory viruses, such as influenza and CoVs, which induce diseases associated with excessive immune signalling.









