Leonor Kremer
Group Leader
Research summary
Our main research interest is to understand how chemokines mediate the interaction between tumour cells and their microenvironment, and how they participate in the control of tumour growth and progression. We study the contribution of chemokine receptors to cancer cell physiology, and evaluate their potential as antitumor targets.
Publications
Perez-Zsolt D, Erkizia I, Pino M, García-Gallo M, Martin MT, et al. Anti-Siglec-1 antibodies block Ebola viral uptake and decrease cytoplasmic viral entry. Nat Microbiol 2019; 4: 1558-1570.
Bárcena C, Aran G, Perea L, Sanjurjo L, Téllez É, et al. CD5L is a pleiotropic player in liver fibrosis controlling damage, fibrosis and immune cell content. EBioMedicine 2019; 43:513-524.
Somovilla-Crespo B, Martín Monzón MT, VelaM, Corraliza-Gorjón I, Santamaria S, Garcia-Sanz JA, Kremer L. 92R Monoclonal Antibody Inhibits Human CCR9+ Leukemia Cells Growth in NSG Mice Xenografts. Front Immunol. 2018; 9:77.
Funding


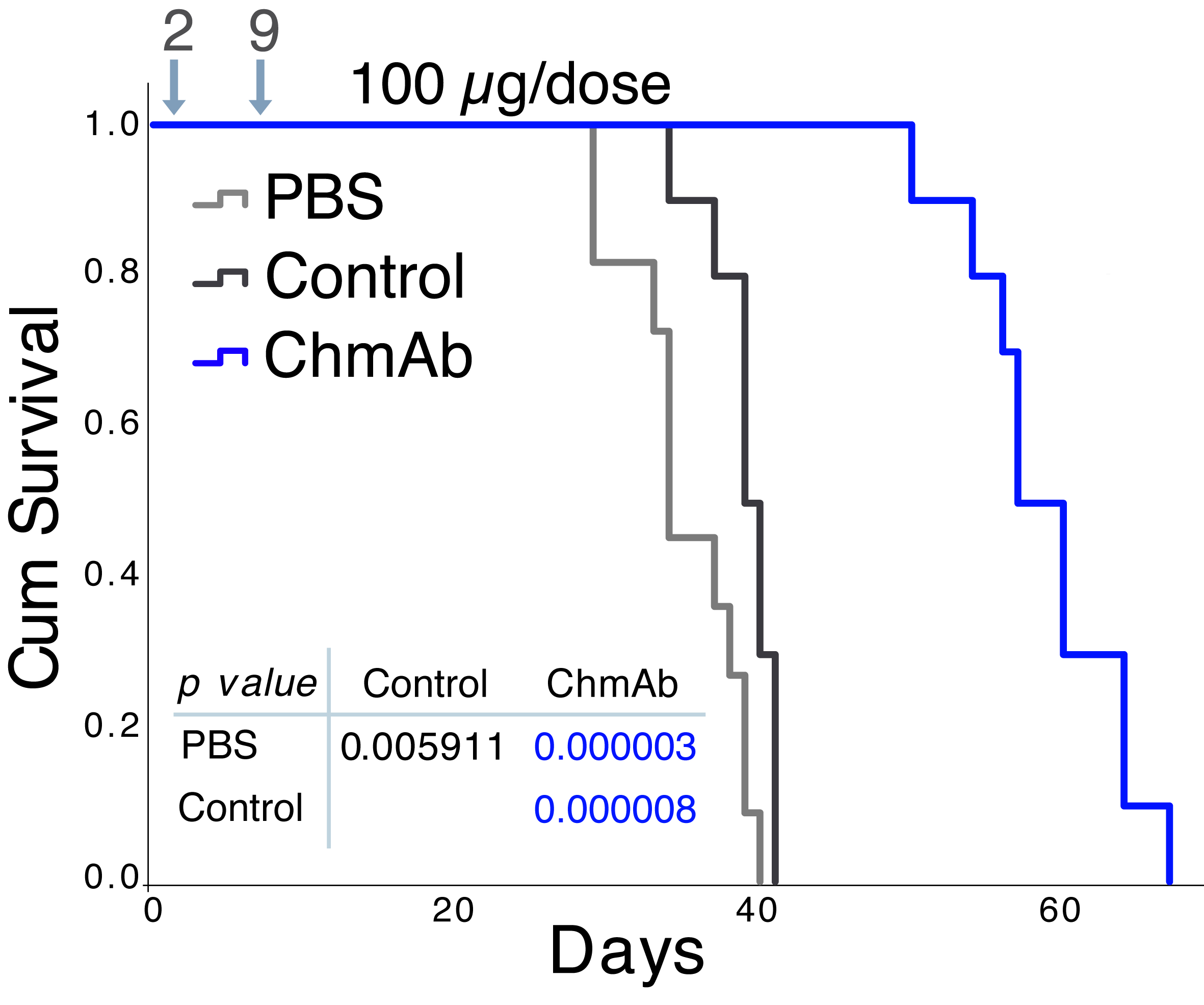
Our group studies the role of chemokines and their receptors in tumour progression and metastasis, since they are involved in tumour cell survival and proliferation, tumour-associated angiogenesis and the antitumour immune response. There is growing interest in the development of new antibody-based immunotherapies for cancer treatment. We have generated a panel of mouse monoclonal antibodies (mAb) specific for the human CCR9 receptor, which is overexpressed in different haematological malignancies. Two antibodies that were selected for their efficacy in reducing the growth of human CCR9+ tumours in different immunodeficient mouse models have been protected by an international patent and have been licensed to SunRock Biopharma. Chimeric and humanised variants of these antibodies also effectively inhibit tumour growth. Recently, using the CRISPR/Cas9 system, we have generated tumour cell lines with modified variants of CCR9, which are being used in ongoing experiments on animal models to evaluate whether the candidate antibodies for clinical use exhibit any off-target side effects.
With the aim to generate antibody cocktails that can simultaneously attack different molecular targets on leukaemia cells, we generated mAb against surface antigens present on human T-cell acute lymphoblastic leukaemia cells. Several of them strongly reduce tumour size in animal models. Using proteomic techniques, we are selecting those mAb that recognise surface cell molecules with a greater therapeutic potential.
In collaboration with different research groups, we are also generating and evaluating mAb that can be used to modulate the immune response in other pathologies. We have contributed to analyse the role of the CCL1-CCR8 axis in atherosclerosis, to study CD5L in liver fibrosis and to generate new tools for inhibiting Ebola and HIV-1 viral uptake. We have also generated mAb against SARS-CoV-2 that are being evaluated as potential therapeutic agents for the treatment of COVID-19.
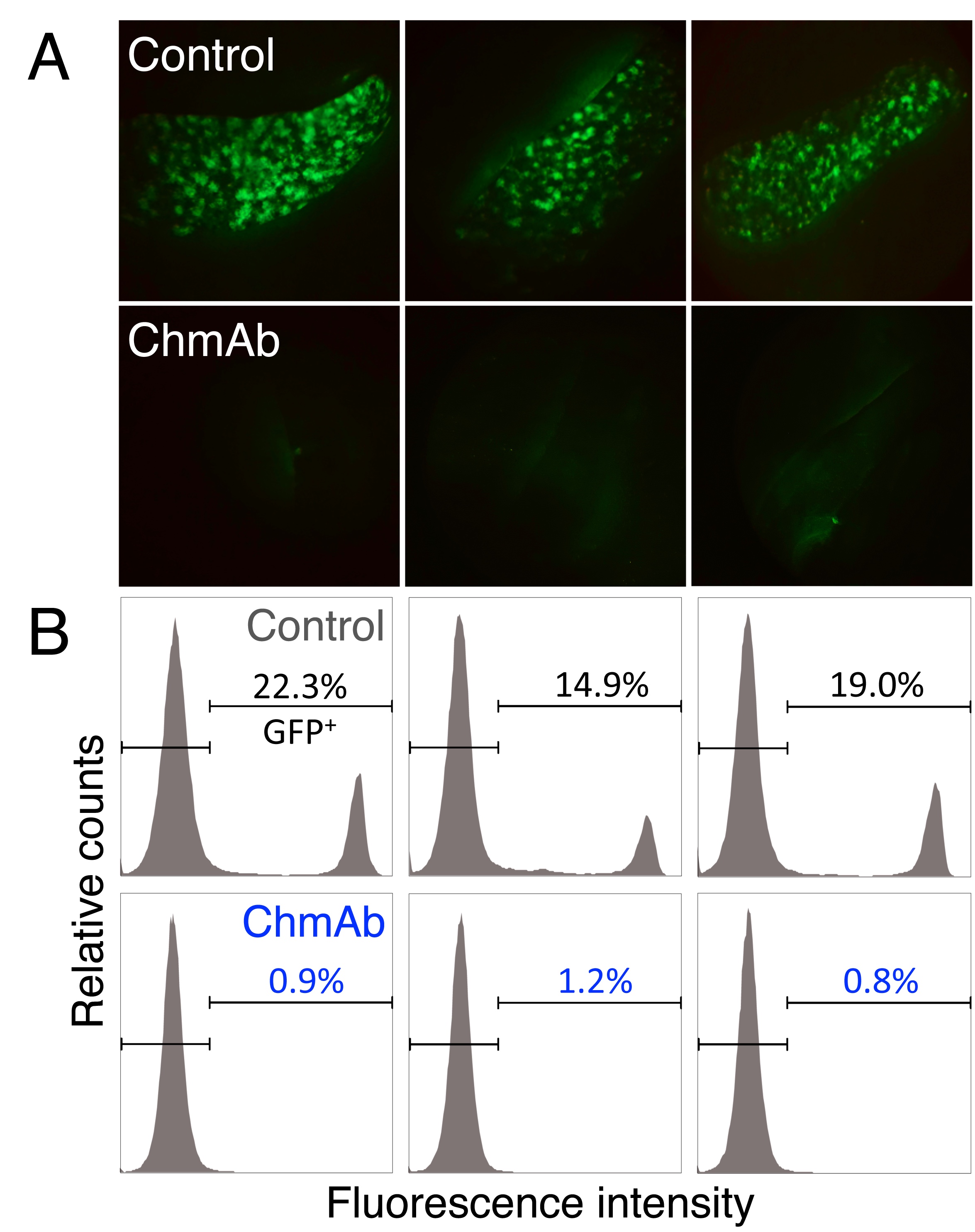
Figure legend: A. Stereomicroscopic images of a representative spleen from each treatment group, where the accumulation of tumour cells (MOLT-4-GFP+) in the isotype-control treated animals but not in the ChmAb-treated group, could be observed. Data from 3 mice of each 10 mice group are shown. B. Flow cytometry analyses of mouse bone marrow showing the fraction of MOLT-4-GFP+ cells from isotype control and ChmAb-treated animals. Data from 3 mice of each 10 mice group are shown.








