José F. Rodríguez
Group Leader
Research summary
Our main virus model is the infectious bursal disease virus (IBDV), the etiological agent of an acute immunosuppressive disease affecting domestic chickens. Our group is mainly interested in birnavirus structure and morphogenesis; and in the molecular basis for IBDV virulence.
Publications
Broto L, Romero N, Méndez F, Diaz-Beneitez E, Candelas-Rivera O, et al. Type I Interferon acts as a major barrier to the establishment of infectious bursal disease virus (IBDV) persistent infections. J Virol. 2020 Dec 16:JVI.02017-20.
Ferrero DS, Busnadiego I, Garriga D, Guerra P, Martín MT et al. Structure and dsRNA-binding activity of the Birnavirus Drosophila X Virus VP3 protein. J Virol. 2020 Nov 25:JVI.02166-20.
Cubas-Gaona LL, Diaz-Beneitez E, Ciscar M, Rodríguez JF, Rodríguez D. Exacerbated apoptosis of cells infected with infectious bursal disease virus (IBDV) upon exposure to Interferon alpha (IFN-α). J Virol 2018; 92: e00364-18.
Méndez F, Romero N, Cubas LL, Delgui L, Rodríguez D, Rodríguez JF. Non-lytic egression of infectious bursal disease virus (IBDV) particles from infected cells. PLoS One 2017; 12: e0170080.
Lago M, Rodríguez JF, Bandín I, Dopazo CP. Aquabirnavirus polyploidy: A new strategy to modulate virulence? J Gen Virol 2016; 97:1168-1177
Landajuela A, Hervás JH, Antón Z, Montes LR, Gil D, Valle M, Rodriguez JF, Goñi FM, Alonso A. Lipid geometry and bilayer curvature modulate LC3/GABARAP-mediated model autophagosomal elongation. Biophys J 2016; 110: 411-22
 The Birnaviridae family comprises naked icosahedral viruses with bipartite dsRNA genomes that infect a wide variety of animal species including insects, aquatic fauna and birds. Despite its socioeconomic importance, critical aspects of birnavirus molecular biology are poorly characterized. Our main virus model, infectious bursal disease virus (IBDV), is the aetiological agent of an acute immunosuppressive disease that affects juvenile domestic chickens and causes heavy economic losses to the poultry industry world-wide (http://www.oie.int/eng/maladies/en_classification2007.htm?e1d7). The main goal of our laboratory is to better understand the virus-host interactions that underlie birnavirus pathogenesis, and to use this knowledge to develop sustainable strategies for birnavirus-borne disease control.
The Birnaviridae family comprises naked icosahedral viruses with bipartite dsRNA genomes that infect a wide variety of animal species including insects, aquatic fauna and birds. Despite its socioeconomic importance, critical aspects of birnavirus molecular biology are poorly characterized. Our main virus model, infectious bursal disease virus (IBDV), is the aetiological agent of an acute immunosuppressive disease that affects juvenile domestic chickens and causes heavy economic losses to the poultry industry world-wide (http://www.oie.int/eng/maladies/en_classification2007.htm?e1d7). The main goal of our laboratory is to better understand the virus-host interactions that underlie birnavirus pathogenesis, and to use this knowledge to develop sustainable strategies for birnavirus-borne disease control.
During the last few years, our work has been mainly devoted to understanding the interaction between IBDV and host cells and the impact of the innate antiviral response on both virus-induced pathogenesis and the establishment of persistent IBDV infections. Our studies have unveiled the chief role of type I interferons on both phenomena, showing that the activation of the JAK-STAT pathway early after IBDV infection leads to a massive apoptotic response contributing to the deadly cytokine storm that destroys the bursal tissue and eventually finish off infected birds. Conversely, we have shown that the genetic inactivation of the JAK-STAT pathway significantly reduces IBDV cyto-pathogenicity and largely enhances the susceptibility of infected cells to sustaining long-term, productive, persistent infections.
Currently, in collaboration with Dr. Soubies’s group (OIE Reference Laboratory for Gumboro Disease, French Agency for Food, Environmental and Occupational Heath Safety, Ploufragan, France) we are further dissecting mechanisms responsible for the establishment of persistent infections in infected chickens, a major IBDV biological trend likely playing a major role on IBDV dissemination and reemergence. We are particularly interested in deciphering the role of cell/virus genetic elements (e.g. micro-RNAs and defective virus genomes) within this phenomenon. Additionally, we focus on the characterization of used by IBDV-encoded polypeptides, namely VP3 and VP5, involved in the evasion of innate antiviral cell responses. Finally, we are committed to further knowledge about the IBDV replication process, specifically on the morphogenesis of replicative complexes and progeny assembly and virus egress mechanisms.
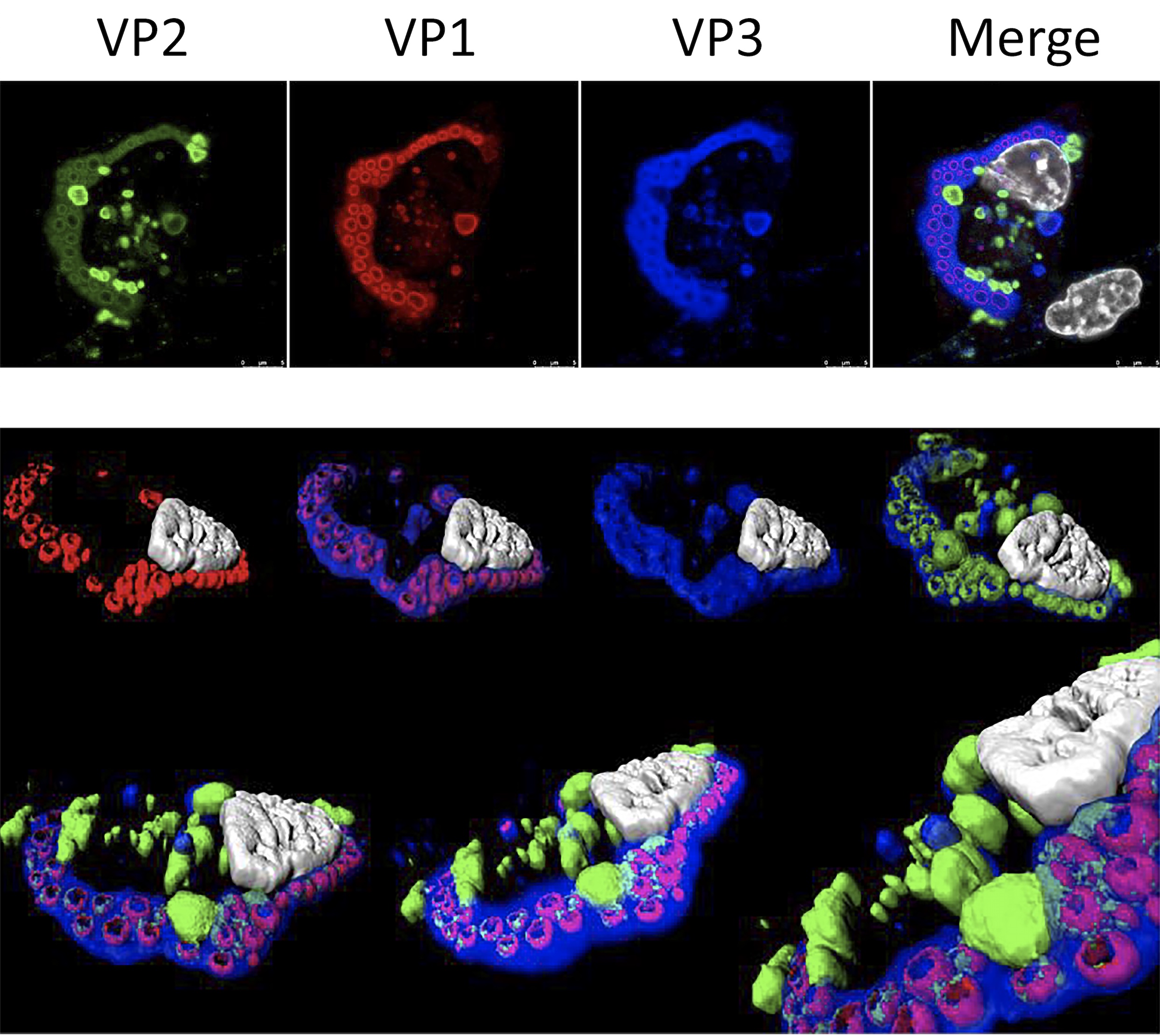
Characterization of IBDV replication complexes. Upper panels correspond to a single confocal plane captured from an IBDV-infected QM7 cell immuno-stained with antibodies specifically recognizing the three structural virus polypeptides, i.e. VP1 (red), VP2 (green) and VP3 (blue). The cell nucleus, stained with DAPI, is shown in white. Lower panel show images from a 3D reconstruction, generated with confocal planes captured along the z-axis, showing the presence of quasi-spherical nucleation domains embedded within large viroplasms. The compact superstructures, exclusively stained with VP2 antibodies, adjacent to viroloplasms, correspond to aggregates formed by tightly packaged IBDV virions.








