Juan Carlos Alonso
Group Leader
Silvia Ayora
Group leader
Research summary
Our group is devoted to analyze the factors that govern genetic stability in bacteria, using Bacillus subtilis as the model.
Publications
Carrasco, B. Serrano E, Martín-González A, Moreno-Herrero F, Alonso JC. Bacillus subtilis MutS modulates RecA-mediated DNA strand exchange between divergent DNA sequences. Front Microbiol 2019, 10: 237.
Serrano E, Ramos C, Ayora S, Alonso JC. Viral SPP1 DNA is infectious in naturally competent Bacillus subtilis cells: inter- and intramolecular recombination pathways. Environ Microbiol. 2020, 22(2):714-725.
Torres R, Alonso JC. Bacillus subtilis RecA, DisA, and RadA/Sms interplay prevents replication stress by regulating fork remodeling. Front Microbiol 2021, 12:766897.
Serrano E, Torres R, Alonso JC. Nucleoid-associated Rok differentially affects chromosomal transformation on Bacillus subtilis recombination-deficient cells. Environ Microbiol 2021, 23(6):3318-3331.
Serrano E, Ramos C, Alonso JC, Ayora S. Recombination proteins differently control the acquisition of homeologous DNA during Bacillus subtilis natural chromosomal transformation. Environ Microbiol 2021, 23(1):512-524. doi: 10.1111/1462-2920.15342.
Ramos C, Hernández-Tamayo R, López-Sanz M, Carrasco B, Serrano E, Alonso JC, Graumann PL, Ayora S. The RecD2 helicase balances RecA activities. Nucleic Acids Res 2022, 50(6):3432-3444 (2022) doi: 10.1093/nar/gkac131.
Our research focuses on the study of the molecular mechanisms that secure genomic stability, promote horizontal gene transfer and control cell proliferation using Bacillus subtilis (a representative bacteria of the Firmicutes phylum) as a model. The work is divided in three main subjects.
Natural competence. Natural transformation is a mechanism for the horizontal transfer of genes encoded entirely by the cell receptor. Competent cells capture the exogenous DNA, and may integrate into the host chromosome by homologous recombination, mediated by RecA recombinase. We analyze: i) the mechanisms and recombination proteins responsible for RecA-mediated DNA integration of self (intraspecies) and non-self (interspecies) DNA, ii) the impact of chromosome structure in the acquisition of interspecies DNA, and (iii) how extrachromosomal genetic elements, which contribute to the acquisition of antibiotic resistance, can be acquired by natural competence.
Homologous recombination and damage tolerance. When the replisome or a RNA polymerase elongation complex encounter DNA damage, replication and transcription stall, leading to traffic conflicts. Then recombination and repair proteins become essential to maintain genom stability. (i) We analyze how the RecA recombinase is modulated by recombination proteins (RarA and RecD2) that are recruited at active replication forks, (ii) How the DisA damage sensor protein modulates the activity of recombination helicases (RadA/Sms, RecG, and RuvAB) that act upon replication stalling; and iii) We analyze DNA helicases and nucleases that contribute to the resolve transcription-replication conflicts.
Segregational stability. We study how toxin-antitoxin and partition systems contribute to segregation stability using plasmid pSM19035 as a model.
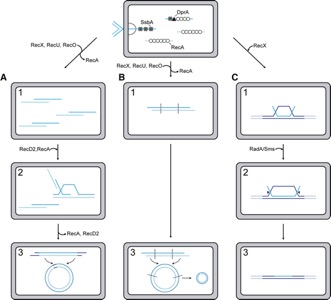
Figure 1. Mechanisms of DNA acquisition by recombination in competent B. subtilis cells. RecA recombinase, with the help of the DprA-SsbA mediators, filaments on the incoming linear SsbA-coated ssDNA. (A) fragmented ssDNA of 12,000- to 16,000-nt in length enters into competent cells, and from these fragments a complete viral SPP1 genome (44.1 kb) is reconstituted by recombination. (B) During plasmid transformation, a similar annealing reaction as in A occurs, but here the DNA molecule has a size higher than the unit-length plasmid molecule, and an intramolecular recombination circularizes the oligomeric plasmid. (C), In chromosomal transformation, homology (intraspecies) or homeology (interspecies) with the host chromosome exists. The RecA nucleoprotein filament efficiently searches for this homology, and when it is found, RecA·ATP catalyses strand invasion and integration of the uptaken DNA.









