Ana Cuenda
Group Leader
Research summary
The aim of our group is to understand the role of p38MAPK proteins in physiology and disease. They regulate diverse cellular functions in normal conditions and in response to environmental stress, infection and proinflammatory cytokines. Moreover, they become deregulated in several human disease situations such as oncogenic transformation and inflammation, making them promising targets for new therapeutic avenues.
Publications
Shabardina V, Romero Charria P, Bercedo Saborido G, Diaz- Mora E, Cuenda A, Ruiz-Trillo I, Sanz- Ezquerro JJ. Evolutionary analysis of p38 stress-activated kinases in unicellular relatives of animals suggests an ancestral function in osmotic stress. Open Biology 2023, 13(1): 220314
Escós A, Martín-Gómez J, González-Romero D, Díaz-Mora E, Francisco-Velilla R, et al. TPL2 kinase expression is regulated by the p38γ/p38δ-dependent association of aconitase-1 with TPL2 mRNA. Proc Natl Acad Sci USA 2022 119(35):e2204752119.
Fajardo P, Taskova M, Martín-Serrano MA, Hansen J, Slott S, et al. p38γ and p38δ as biomarkers in the interplay of colon cancer and inflammatory bowel diseases. Cancer Commun (Lond) 2022, 42(9):897-901.
Fajardo P, Cuenda A and Sanz-Ezquerro JJ. Mouse model of Candidiasis. Methods Mol Biol 2021; 2321:63-74.
Sanz-Ezquerro JJ. and Cuenda A. p38 Signalling Pathway. Int Mol. Sci 2021, 22(3), 1003.
In the last years we have expanded our knowledge on the molecular and cellular mechanisms involved in the inflammatory response in the settings of chronic inflammation leading to tumour development, as occurring in colorectal cancer (CRC) associated to colitis. CRC is the second leading cause of cancer death. Patients with inflammatory bowel disease (IBD), ulcerative colitis or Crohn’s disease are at increased risk of developing CAC; however, our understanding of the interplay inflammation-cancer at the molecular level is limited. We have reported that p38γ/p38δ have a pro-oncogenic function in CRC by regulating the production of inflammatory molecules and miRNAs, in humans. We found that p38γ/p38δ protein can be detected in human plasma, and that p38γ is significantly upregulated in IBD and CRC patients indicating that can mediate inflammation signaling to promote tumorigenesis. Our work suggests that p38γ/p38δ can be useful biomarkers for CRC/IBD diagnostic, as well as potential treatment targets, for colitis and early-stage CRC. Additionally, our data support the role of p38γ/p38δ in promoting tumour growth and give further evidence that one of the mechanisms by which p38γ promotes tumorigenesis is linked to its elevated protein expression and activation, thus increasing inflammation and providing an inflamed tumour environment that would promote tumour growth.
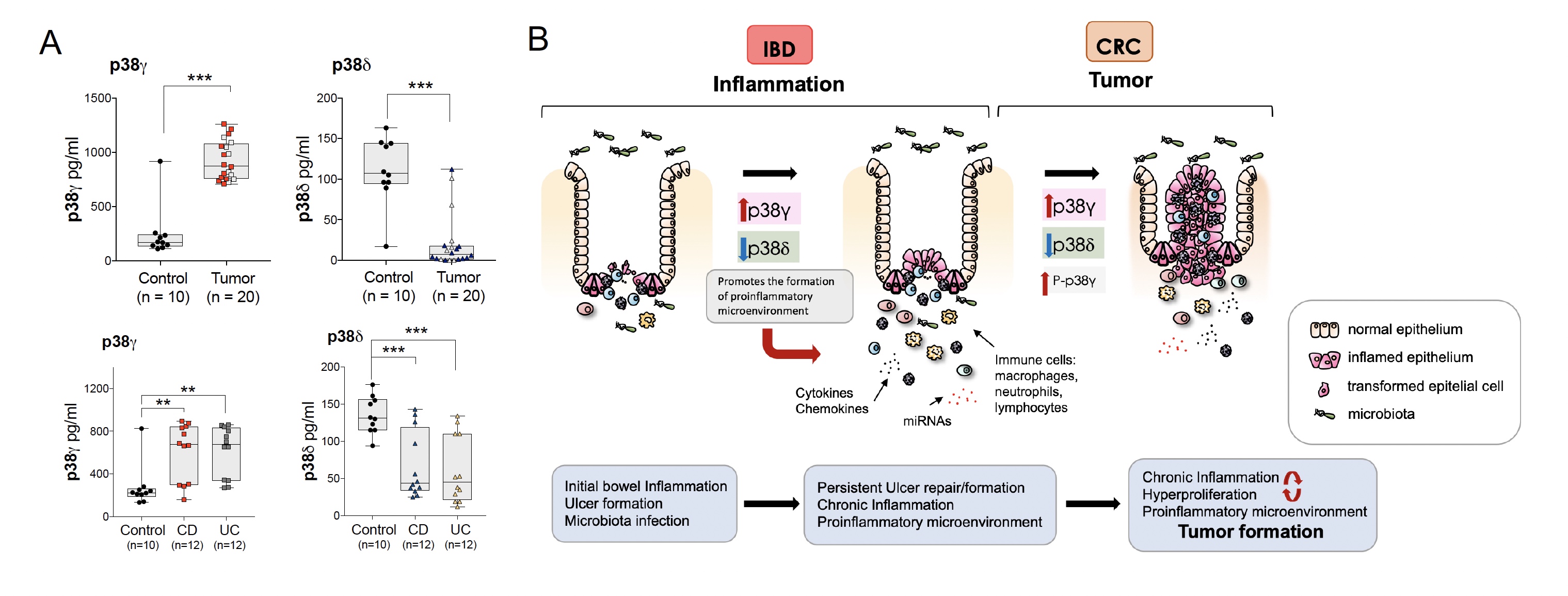
Figure 1. A. p38γ and p38δ expression in the plasma of healthy donors (control), colon cancer patients (tumor) or of IBD (CD and UC) patients. Each dot is a single patient or donor. (Upper panel) p38γ expression in CRC patients with (grey square) or without (red square) IBD; p38δ expression in CRC patients with (grey triangle) or without (blue triangle) IBD. Student’s t-test. **P ≤ 0.01; ***P ≤ 0.001. B. Schematic illustration of the function of alternative p38MAPKs, p38γ and p38δ, in IBD and CRC formation.
We have further investigated the molecular mechanism by which p38γ/p38δ regulate cytokine production and found that these kinases regulate inflammation, in part by controlling the expression of the ERK1/2 upstream kinase, tumour progression locus 2 (TPL2), in myeloid cells. We have demonstrated that TPL2 protein level is regulated by p38γ/p38δ at two different levels: 1) interacting with the TPL2/A20 Binding Inhibitor of NF-κB2 (ABIN2)/Nuclear Factor κB1p105 (NF-κB1p105) complex, increasing TPL2 protein stability; and 2) controlling TPL2 mRNA translation by modulating the repressor function of TPL2 3' Untranslated region (UTR) mediated by its association with aconitase-1 (ACO1).
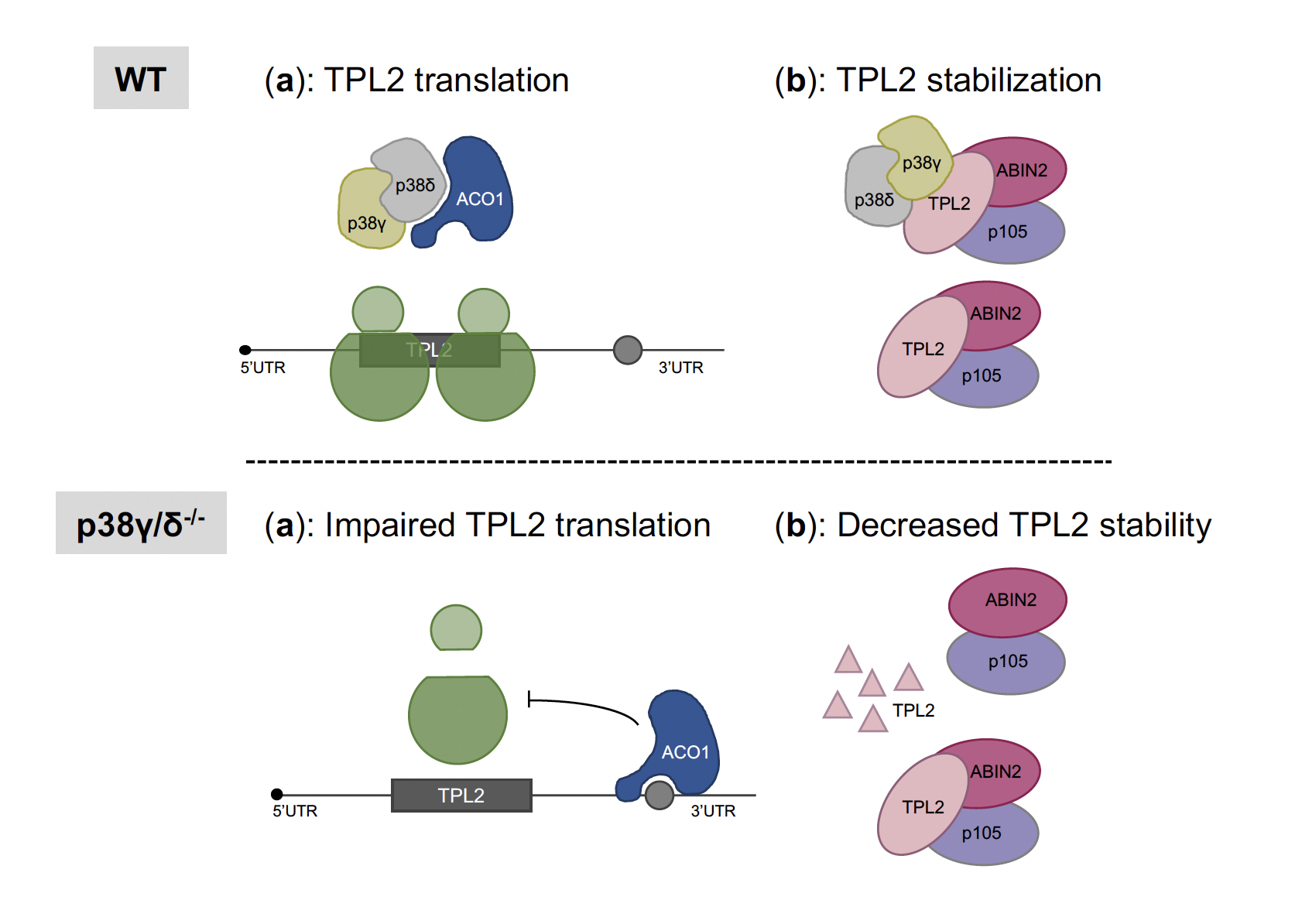
Figure 2. Model for the regulation of TPL2 protein levels by p38γ and p38δ. In WT cells, p38γ and p38δ associate with ACO1 and also with the TPL2/ABIN2/p105 complex. (a) In WT cells, the p38γ/p38δ/ACO1 complex prevents ACO1 from binding to TPL2 3´UTR, and TPL2 mRNA is translated. In p38γ/δ-/- cells, free ACO1 binds to TPL2 3´UTR, and impairs TPL2 mRNA translation. (b) In WT cells, the p38γ/p38δ/TPL2/ABIN2/p105 complex stabilizes TPL2 protein, whereas p38γ/p38δ absence decreases TPL2 stability and increases its degradation.








