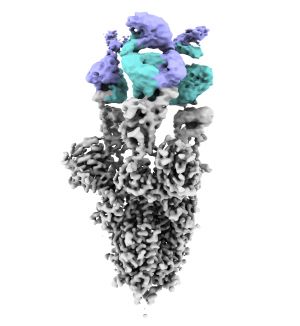
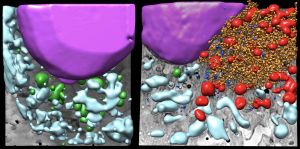
The covid-19 pandemic has affected more than 770 million people and has caused the death of nearly seven million people around the world. Its huge impact on health and global economy has promoted research in the field since 2020, although it is still necessary to understand how this infection makes progress with the aim of finding specific solutions to this pathogen. Now, a team from the Spanish National Research Council (CSIC) and the ALBA Synchrotron publishes in the journal ACS Nano the results obtained after three-dimensional analysis of the interior of an infected cell.
Members of the National Centre for Biotechnology (CNB-CSIC) and the ALBA Synchrotron, the only synchrotron light source in Spain located in Cerdanyola del Vallès (Barcelona), have imaged in three dimensions the interior of human lung epithelium cells, the primary target of the virus, and the severe structural changes caused by SARS-CoV-2 infection.