Microscopía Óptica Avanzada
SERVICIOS

Ana Oña
Head of Advanced Optical Microscopy
FACILITY CONTACT
CNB-CSIC
C/Darwin 3, Madrid 28049
Email: Click here
Tel: 91 585 4661 (432103)
Equipment Calendars
Confocal Stellaris
Confocal SP5
MicroFluor
Confocal SP8
Yoda (WF-TIRFM)
Confocal Stellaris 8
Processing and Analysis
Other documents
The unit offers state-of-the-art infrastructure for widefield, confocal, nanoscopy and mesoscopy through advanced optical microscopy techniques: STED (STimulated Emission Depletion), FLIM (Fluorescence-Lifetime Imaging Microscopy), FCS (Fluorescence Correlation Spectroscopy), DLS (Digital LightSheet), TIRFM (Total Internal Reflection Fluorescence Microscopy) and SMLM (Single-Molecule Localization Microscopy), covering most multi-colour fluorescence imaging studies with fixed and live samples. The equipment and services are available to all CNB staff, as well as to external researchers from the public and private sectors.
The unit establishes collaborations with researchers whose projects require the use of optical microscopy, providing the extensive experience of the facility’s personnel. The scientific-technical staff provides assistance, training and advice throughout the entire workflow: sample preparation, use of equipment, available methodologies, image processing, quantification and analysis procedures.
Cell culture slides, secondary antibody aliquots and probes widely used in fluorescence microscopy applications are also provided.
Applications
Confocal Microscopy Applications:
- Multichannel confocal and/or transmission imaging of living or fixed samples (2D, 3D and 4D imaging).
- Advanced microscopy techniques: STED 3X, FLIM, FCS and DLS.
- Multidimensional in vivo time-lapse experiments.
- High speed confocal microscopy.
- FRET, FRAP, photoactivation, photoswitching, excitation/emission lamda scan, reflection.
- Cellular/subcellular localization and colocalization studies.
- Tile-scan imaging.
Widefield Microscopy Applications:
- Multichannel fluorescence and/or transmission imaging (BF, DIC, phase contrast).
- Multidimensional in vivo time-lapse experiments (wound healing, infection, migration, etc).
- Single, sequential or green/red fluorochromes simultaneous TIRF microscopy for the study of dynamic processes or molecule localization at the cell surface level.
- Single molecule localization microscopy (PALM/STORM).
- Tile-scan imaging.
Leica STELLARIS 8 STED 3X multispectral confocal nano and mesoscopy system
- White laser with excitation range 440 to 790 nm and 405 nm diode.
- Two Power HyD S spectral detectors and two Power HyD X spectral detectors.
- STED/TauSTED 3X nanoscopy module with three depletion lines: 592, 660 and 775 nm.
- FLIM (Fluorecence Lifetime Imaging Microscopy) system.
- FCS (Fluorecence Correlation Spectroscopy) system.
- DLS (Digital LightSheet) mesoscopy system.
- Integrated software module for real-time multidimensional super-resolution multidimensional image detection and processing (Lightning).
- Environmental control camera (Okolab) for time-lapse studies.
Ayuda EQC2021-007293-P financiada por:

Leica TCS SP8 STED 3X multispectral confocal system
- White laser with excitation range 470 to 670 nm and 405 nm diode.
- Three HyD hybrid detectors with time gating.
- STED 3X nanoscopy module with two depletion lines: 592 and 660 nm
Leica STELLARIS 5 multispectral confocal system
- Four laser lines: 405, 488, 561 and 638 nm.
- Three Power HyD S spectral detectors.
- Integrated software module for real-time multidimensional super-resolution multidimensional image detection and processing (Lightning).
Leica TCS SP5 multispectral confocal system
- Seven laser lines: 405, 458, 476, 488, 514, 561 and 633 nm.
- Resonant scanner module for high-speed acquisition.
- Environmental control system for time-lapse studies.
Leica DMi8 S epifluorescence system
- Orca Flash 4.0 sCMOS camera (Hamamatsu).
- LEDs
- TIRFM (Total Internal Reflection Fluorescence Microscopy) module.
- High-power 405, 488, 514, 561 and 638 nm lasers for Single-Molecule Localization Microscopy (PALM/STORM).
- Gemini W-View splitter (Hamamatsu) for simultaneous acquisition with green/red fluorochromes.
- Environmental control camera (Okolab) for time-lapse studies.
Leica DMI6000B epifluorescence microscope
- OrcaR2 monochrome digital camera.
- Environmental control system for time-lapse studies.
Image processing and analysis
Three independent workstations with the software for image processing and analysis: Leica LAS AF, Leica LAS X, Leica LAS X FLIM/FCS, Huygens, Aivia, Imaris, Metamorph, ImageJ/Fiji, Cell profiler.

Personnel
Scientific Coordinator
José María Requejo
Head
Ana Oña
Technicians
Gianluca D’Agostino
Jaime Fernández de Córdoba

Get to know our facility work
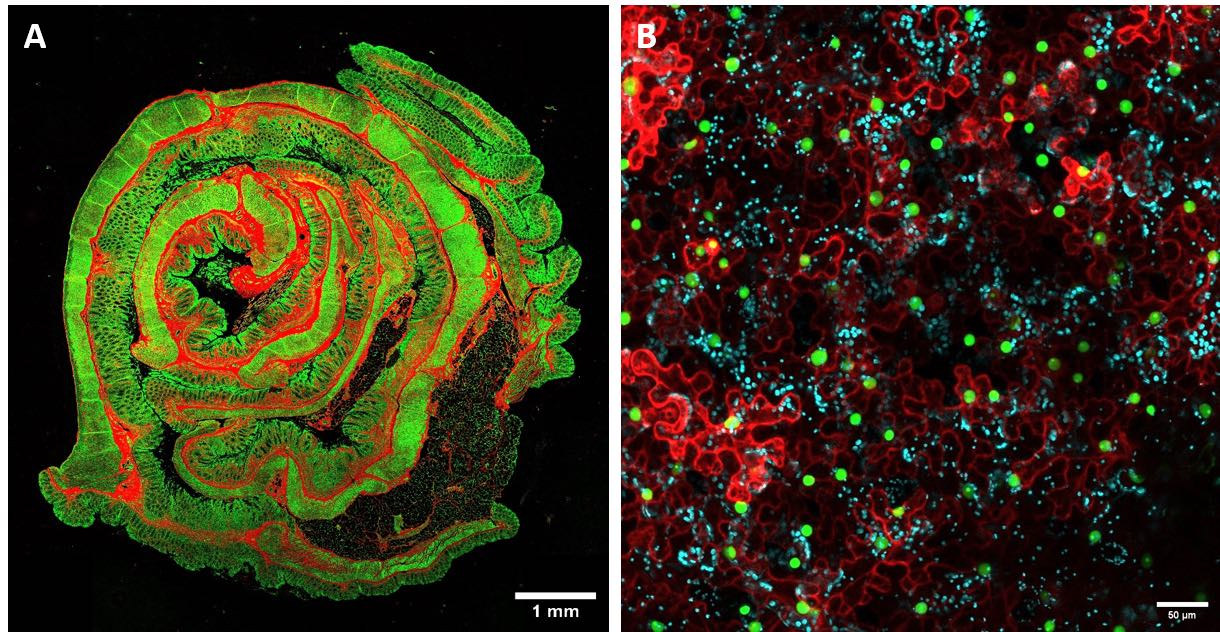
Mesoscopy imaging. (A) Representative mosaic FLIM image of animal tissue (intestine). (B) Representative confocal image of vegetal tissue (leaf).
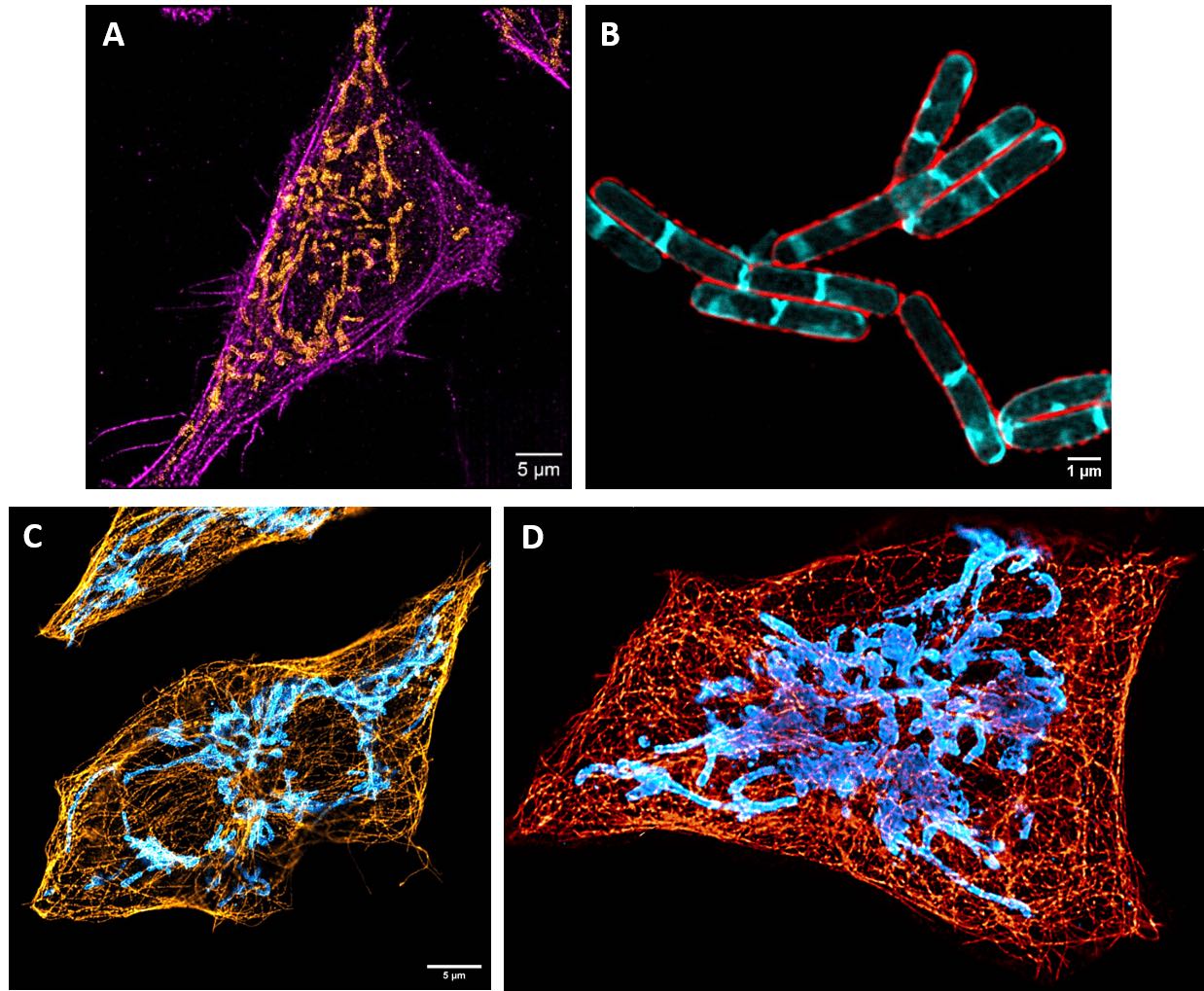
Nanoscopy imaging (A) Representative STORM image of the actin cytoskeleton (magenta) and mitochondria (orange) in human cell. (B) Representative STED image of the cell membrane (red) and the septum (cyan) in bacteria. (C)Representative STED image of the tubulin cytoskeleton (orange) and mitochondria (blue) in human cell. (D) 3D reconstruction of STED images.



