Stem Cells in Health and Disease
RESEARCH GROUPS

Antonio Bernad
Group Leader

Carlos Martínez-A
Group Leader
Research Summary
Our research is dedicated to understanding the intricate molecular mechanisms that govern cell identity and fate within the fields of developmental and stem cell biology. A central focus of our work is the analysis of gene expression patterns, crucial for defining cellular characteristics. We investigate these patterns in both states of homeostasis, representing normal physiological conditions, and in various disease contexts. These diseases include metabolic syndrome, immune system disorders, and the development and progression of various types of cancer.
Research Lines
Our group has defined a minoritarian cardiac subpopulation B-CPC (see figure 1), characterized by the expression of the transcriptional factor Bmi1, enriched in cardiac stem cells (CSC) and cardiac progenitor cells (CPC). B-CPC is a heterogeneous population that can become any of the three main cardiac cell lineages, both in homeostasis and after acute injury. B-CPC are localized in perivascular locations, mainly in some cardiac specialized endothelial domains (low-oxidative stress structures). This low oxidative environment reduces B-CPC mitophagy and slow metabolism, protecting B-CPC from the generalized age-related oxidative stress. This represents in adult heart the endothelial niche, as in other adult stem cell compartments and the intime interaction with endothelium keeps B-CPC in a quiescent status. As previously established in skeletal muscle, we have confirmed a bidirectional regulation; B-CPC secrete high levels of the chemokine CXCL12 to maintain endothelium in their close vicinity and the Dll4 (endothelium) / Notch (B-CPC) pair is critically involved in regulating the equilibrium of the immature state versus the differentiation response.
Additionally, genome-wide screens and multi-omics approaches have identified a number of potential regulators of stem cell function, including genes with established roles in transcriptional regulation, cell growth and differentiation, as well as genes whose function is either largely unknown or remains to be validated in the context of stem cell biology, such as the Dido gene.
The Dido locus is strongly expressed in stem cells, which decreases during development and in somatic cells; its expression is also very high in iPS (induced pluripotent stem cells) generated from somatic cells. Its presence is nonetheless critical for cell survival. Dido, a gene complex found only in higher vertebrates, encodes three splice variants: Dido1 (the smallest of these), Dido2, and Dido3. Dido3, the largest and most broadly expressed isoform, encodes a nuclear protein that recognizes trimethylated histone H3 lysine 4 through its N-terminal PHD domain; it interacts with centrosomes and the synaptonemal complex in somatic and germ cells, respectively. N-terminal truncation of Dido3 (Dido3DNT) provokes aneuploidy, centrosome amplification, increased incidence of hematological myeloid neoplasms, brain abnormalities, behavioral alterations and metabolic syndrome.
All attempts to date to delete Dido or a mutant with the exon XVI (Dido3E16) deleted in mice, show that the deletion is incompatible with life. Interestingly, stem cells derived from Dido3 mutants do not differentiate in vitro but they retain their capacity for self-renewal. Furthermore, it has been shown that differentiation can be recovered by expression of full-length Dido3. Based on these results, we propose that Dido acts as a switchboard between self-renewal and differentiation (see figure 2). As dedifferentiation and self-renewal are hallmarks of cancer, we have also studied the susceptibility of Dido3E16 MEF (mouse embryonic fibroblasts) to oncogenic insults in vitro. Our research on cancer also incorporates bioinformatics analysis of publicly available data.
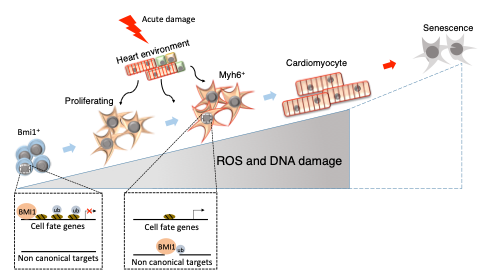
Role of Bmi1 in immature B-CPC versus differentiated cardiomyogenic committed progenitors and further ageing (adapted from Cell Death Differ. 25, 809-822, 2018).
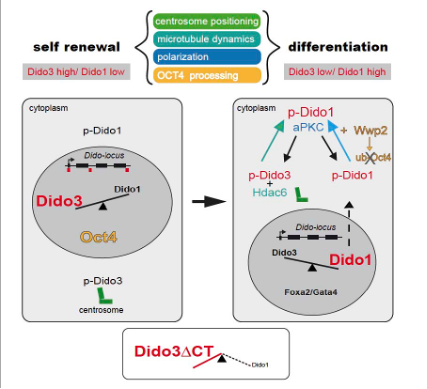
Dido isoforms regulate symmetric and asymmetric cell division of embryonic stem cells (ESC). While Dido3 is involved in centrosome positioning to polarize ESC cells, Dido1 is determinant for their fate identity. Moreover, Dido3 regulates Dido1 expression at the onset of ESC differentiation (from Stem Cell Reports 8, 1062–1075, 2017).
- Currently, we are studying the role of the transcription factor Tbx3 as a relevant intrinsic factor coordinating self-renewal with an equilibrated differentiation.
- Role of Dido in the development of the immune system. We have generated a genetically modified mouse in which the Dido3 carboxy-terminal deletion Dido3E16 is induced in somatic cells by introduction of a floxed exon 16 and the gene of Cre recombinase expressed under the control of VAV1. We will use the mouse mutants to study the immune system development.
- Altered Dido expression is observed in a systemic mouse model of metabolic syndrome and has been identified as a potential marker of obesity in human patients (UK Biobank). We are in studying the function of Dido3E16 in the liver and adipose tissue, key organs involved in metabolic regulation, using genetically engineered tissue-specific mice under different dietary conditions. These mice models will allow us to dissect the role of the liver and fat tissue to coordinate the insulin sensitivity (potentially regulating glucose uptake in the skeletal muscle), lipid metabolism, and obesity-induced inflammation.
- We previously identified the role of Dido in EMT (epithelial-mesenchymal transition) and MET (mesenchymal-epithelial transition); we plan now to study the role of Dido in pancreatic cancer. We have bred the Dido3E16 mice with other mice bearing inducible Ptf1a, Kras, to develop a novel mouse model for pancreatic cancer, in which DIDO3 deficiency can be induced, in the presence or the absence of p53, in adult stages.
Publications
Group Members

Group Leaders
Antonio Bernad
Carlos Martínez-A
Project Leader
Thierry Fischer
Staff Scientists
Julio Gutiérrez
Tirso Pons
Postdoctoral Researcher
José Luis Torán
PhD Candidate
Marina Higuera
Lab assistants
María Ángeles García-López
Ainhoa Sánchez de Diego
Funding
Our research is funded by national and international institutions as indicated below. For more details, please check the general Funding Section at the CNB website.



News
Real-space heterogeneous reconstruction, refinement, and disentanglement of CryoEM conformational states with HetSIREN
Nat Commun. 2025 Apr 22;16(1):3751. Herreros D, Mata CP, Noddings C, Irene D, Krieger J, Agard DA, Tsai MD, Sorzano COS, Carazo JM. Abstract Single-particle analysis by Cryo-electron microscopy (CryoEM) provides direct access to the conformations of...
Ceftazidime-avibactam use selects multidrug-resistance and prevents designing collateral sensitivity-based therapies against Pseudomonas aeruginosa
Nat Commun. 2025 Apr 9;16(1):3323. Hernando-Amado S, Gomis-Font MA, Valverde JR, Oliver A, Martínez JL Abstract Ceftazidime-avibactam is a β-lactam/β-lactamase inhibitor combination restricted for the treatment of multidrug-resistant infections of Pseudomonas...
El Centro Nacional de Biotecnología estrena nueva imagen para reflejar una etapa de renovación y evolución
El nuevo logotipo está compuesto por una espiral que representa una hélice de ADN vista desde arriba, inspirada en la emblemática “Fotografía 51” de Rosalind Franklin, clave para desentrañar su estructura La presentación de esta imagen coincide con el Día del ADN, una...



