Chloroplast Protein Quality Control
RESEARCH FELLOW

Pablo Pulido
Atracción de Talento Fellow
Research Summary
Protein quality control (PQC) systems are formed by chaperones and proteases that together regulate the correct folding and activity of proteins in every compartment of the cell, including chloroplasts. Misfolded or aggregated proteins are either recycled by chaperones (such as DNAJ and HSP70) or degraded by proteases (such as CLP) in chloroplasts. In our group we aim to elucidate and engineer the molecular machinery that protect proteins in chloroplasts allowing plants to cope with adverse environmental conditions such as high temperature or drought. These abiotic stresses are the leading cause of yield loss in crops in the current context of climate change.
Research Lines
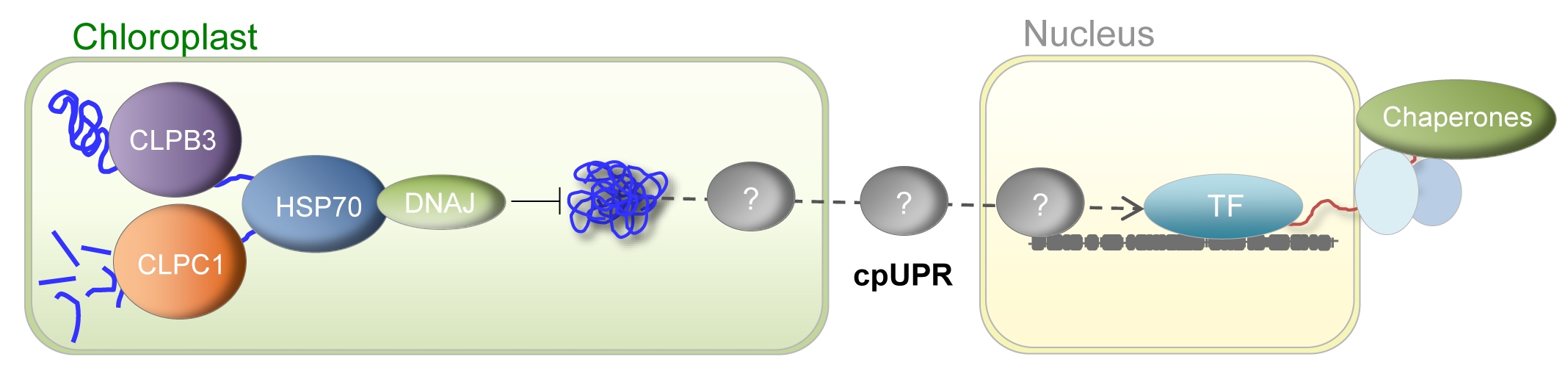
Chloroplasts are the organelles that define plants and the unique site of photosynthesis, the only significant mechanism of energy input into the biosphere. Adverse environmental conditions including high temperatures, cold, drought, and salinity cause protein stress and aggregation in plant chloroplasts. Recycling of damaged proteins is achieved by the action of molecular chaperones but, when recycling is not possible, toxic aggregated proteins have to be degraded by the action of proteases to avoid damage to the organelle. Chaperones and proteases act coordinately and constitute protein quality control (PQC) systems that are required for organismal survival. In chloroplasts, similar mechanisms ruled by the chaperone HSP70 survey for maintaining protein homeostasis (proteostasis).
In our group, we have unveiled a novel synergistic collaboration between the chaperone HSP70 and the protease CLP in preventing the accumulation of protein aggregates. In these synergistic interaction DNAJ proteins are key factors that provide specificity to the system, adaptors that recognise unfolded substrates and transfer them to the HSP70 for refolding, often in cooperation with CLPB3. However, when refolding is not possible, the DNAJ/HSP70 transfer substrates to CLPC1, a subunit of the CLP protease for degradation. The accumulation of protein aggregates unleashes a chloroplast-to-nucleus retrograde signalling pathway that specifically activates the transcription of chaperones at the nucleus, the chloroplast unfolded protein response (cpUPR).
In our goal to design new strategies to engineering plant stress tolerance, we are currently engaged in the study of three interconnected objectives:
– To identify novel substrates of DNAJ proteins in chloroplasts.
– To untangle the specificity of the different CLPC adaptors.
– To unveil components of the cpUPR signalling pathway.
Publications
Group Members
Group Leader
Pablo Pulido
Postdoctoral Researchers
Rocío Álvarez Aragón
Bran López Luengo





