Structure and Function of Molecular Chaperones
RESEARCH GROUPS

José María Valpuesta
Group Leader
Research Summary
Our main goal is to understand the relationship between structure and function of different nanomachines, in particular those formed by molecular chaperones, cochaperones and their protein clients. For this, we use various biophysical techniques, in particular microscopy techniques such as single particle cryoelectron microscopy, cryoelectron tomography, cryocorrelative microscopy, combined with advanced optical microscopy and single molecule optical spectroscopy techniques (such as single-molecule tracking and Fluorescence Correlation Spectroscopy).
Structure of the human CCT (Chaperonin Containing TCP-1), and its conformational cycle. PDB: 6QB8, 4V8R.
Research Lines
We use different biophysical techniques, mostly cryoelectron microscopy (cryoEM), to study the structure and function of different macromolecular complexes, in particular those formed by molecular chaperones, a group of proteins involved in cell homeostasis through two opposite functions, protein folding and degradation. These two cellular processes are carried out through the transient formation of complexes between different chaperones and cochaperones, acting like an assembly line and making this process more efficient.
Our main goal is the structural characterization, at the highest possible resolution of some of these complexes, using as a main tool state-of-the-art cryoEM and image processing techniques. We are particularly interested in locating the binding site of different CCT modulators, on studying the role of molecular chaperones in protein degradation and on developing biotechnological tools derived from human chaperones.
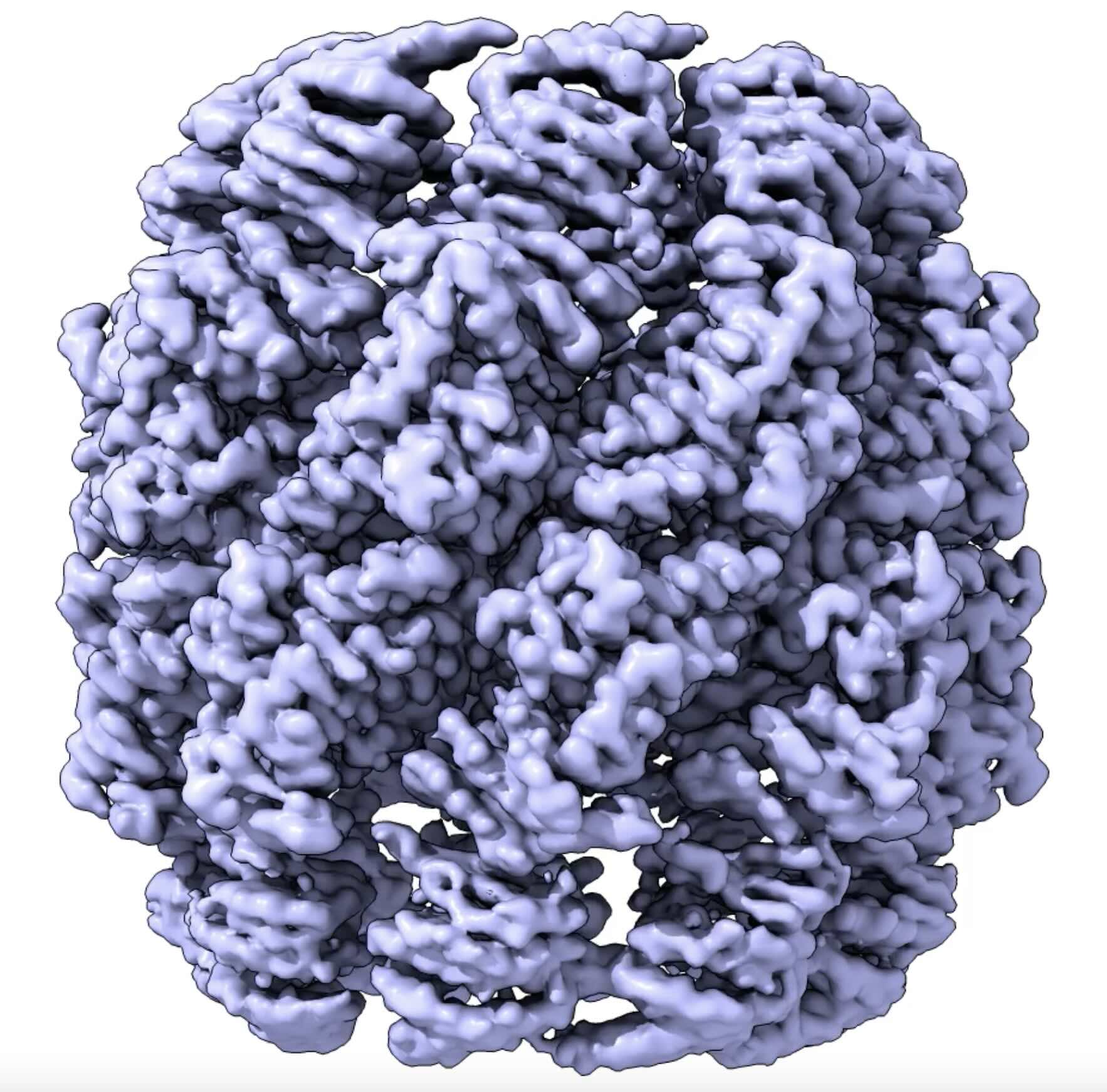
Structure of the synthetic chaperonin Poly-CCT5, a derivative of the human CCT with potential for biotechnological applications.
Structure of the human proteasome, the key of protein degradation in the cell. PDB: 6MSB.
We also aim to study the implication of different chaperones in the regulation of complex cellular events from a structural point of view, as the immune synapse. For that we are implementing correlative approaches to locate and resolve molecular events in a native cellular context.
Additionally, we are interested in the quantitative study of molecular events at the membrane interface, focusing on the role of the membrane physical properties in the functional outcome of molecular interactions. Our toolbox majorly includes advanced optical microscopy and single molecule optical spectroscopy techniques (such as single-molecule tracking and Fluorescence Correlation Spectroscopy).
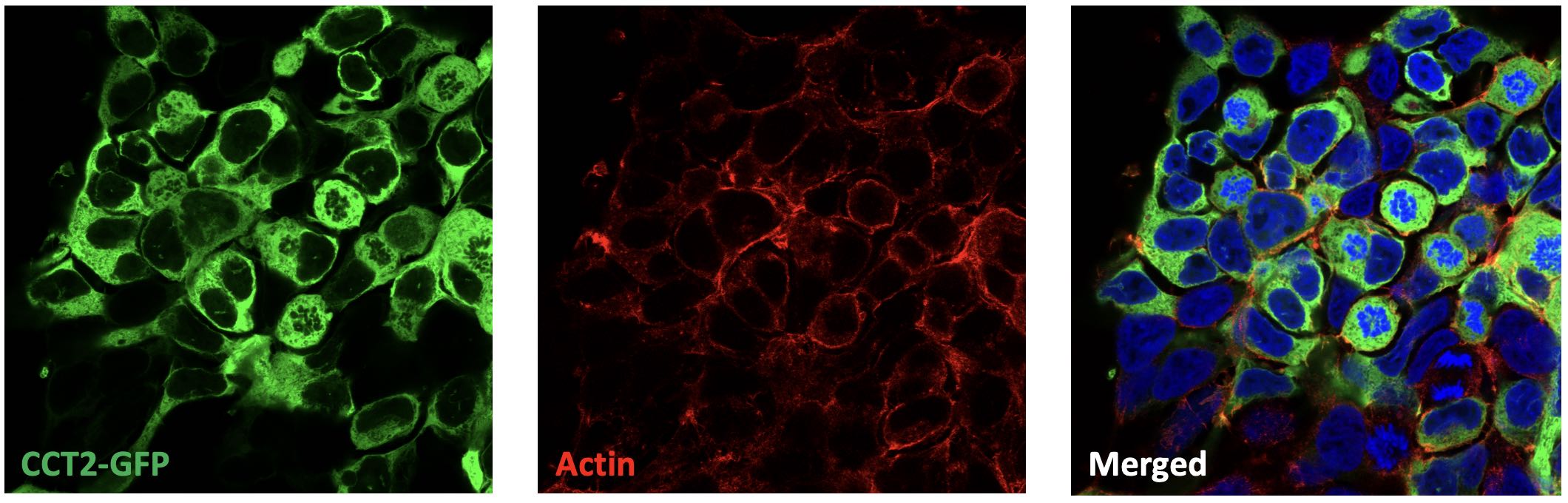
The immunofluorescence images show partial colocalisation of CCT2-GFP (green) with actin (red) in cells, with nuclei stained using DAPI (blue). Actin was visualized using phalloidin, and the images were 2D projections from confocal microscopy.
Publications
Group Members
Group Leader
José María Valpuesta
Jorge Cuéllar
Project leader
Ana Cuervo
Olivia Muriel
César Santiago
Staff Scientist
Javier Rodríguez
Lab assistant
Virginia Rodríguez
PhD candidates
Javier Collado
Jorge Gutiérrez Seijo
Marta Huerta
Carmen Majano
Jimena Muntaner
Sergio Pipaón
Beatriz Sancho

Funding
News
Eva Nogales obtiene una de las cátedras JAE Chairs del CSIC y desarrollará su proyecto en el CNB
31 Mayo 2024 Los proyectos JAE Chairs, otorgados ex aequo, se centran en la mejora de la criomicroscopía electrónica y en el diseño de materiales moleculares para obtener soluciones energéticas innovadoras JAE Chairs apoya iniciativas que generen impacto en el sistema...
Arranca EvaMobs, un proyecto europeo con participación del CNB-CSIC para el desarrollo de nuevos antivirales
7 de Marzo 2024 En este proyecto, financiado por el programa Horizonte Europa, un consorcio de socios con la participación del CNB-CSIC, se ha introducido en una novedosa estrategia de desarrollo de antivirales. En la actualidad, los antivirales de que disponemos se...








