Regulation of inflammation and autoimmunity by p21 and mitochondrial ROS
RESEARCH GROUPS

Dimitrios Balomenos
Group Leader
Research Summary
We showed that p21 suppresses autoimmunity and its overexpression has a therapeutic effect on disease by regulating memory T cell responses and IFN-γ production in lupus-prone mice. Mitochondrial Reactive Oxygen Species (mROS) controls IFN-γ production by memory-like T cells and IFN-γ hyperproduction in autoimmune mice. Moreover, p21 attenuates innate immunity as p21 deficiency increases LPS-induced NF-κB activation and p21 regulation is key in orchestrating M1 to M2 responses. Absence of p21 increases NF-κB activity, deregulation of M1 to M2 plasticity and p21KO mice susceptibility to inflammation and sepsis. The mechanism that regulates and NF-κB–dependent plasticity remains undefined. However, our recent research points to p21 as modulator of mROS, which attenuates NF-κB activity and ultimately immune overactivation.
Research Lines
Our group has first established in past studies that independently of its cell cycle inhibitory capacity p21 is a negative regulator of immune activation and specifically T cell and macrophage function, and it might control other immune cell activity. Other groups confirmed our findings in a variety of models.
A broader objective of our laboratory is to discover the mechanisms by which p21 attenuates T cells and macrophage activation and expand our studies in other forms of immunity and inflammation. Our actual main goal is to verify a relationship between p21 and mitochondrial ROS (mROS) which has emerged as a regulator of the immune response
T cells mROS and p21
With regard to T cells, we have established that mROS controls the production of IFN-γ from previously activated T cells and we have verified the reduced production of IFN-γ through different mROS inhibition strategies. (Rackov et al. 2022)). Mitochondrial reactive oxygen is critical for IL-12/IL-18-induced IFN-γ production by CD4+ T cells and is regulated by Fas/FasL signaling.
Our current work indicates that p21 modulates mROS and IFN-γ production by memory T cells, corroborating our published data and showing that p21 overexpression tempers autoreactive T cells and IFN-γ production.
Macrophages mROS and p21
In macrophages p21 modulates NF-κB activation but also allows polarization of M1 to M2 macrophages (Rackov et al. 2016). In order to study the mechanism by which p21 regulates macrophage activation through mROS, we first examined the kinetics of mROS production and its impact on the TLR4 activation pathway in stimulated macrophages.
We have established that after activation of macrophages via LPS, early mROS signaling promoted the initial activation of NF-κB, MAPK and the production of proinflammatory cytokines, events that were reduced by inhibition or mROS extinction. Although it is generally believed that NF-κB activation drives mROS production, we verified that mROS promotes NF-κB activation. These mechanistic approaches are described in Figure 1.
We discovered a role for p21 in the activation of mROS and in the response of these cells. We have verified a greater production of mROS by p21KO macrophages compared to wt macrophages, which leads to increased activity of NF-κB and production of cytokines. The proposed mechanism represents p21 as a primary regulator of mROS which controls of TRAF-6 ubiquitination and initiates NF-κB activity.
Our future goals are directed in discovering the exact mechanisms that define the interactions between mROS, TRAF-6 ubiquitination and p21 in immunity, and design therapeutic approaches for inflammatory and autoimmune syndromes.
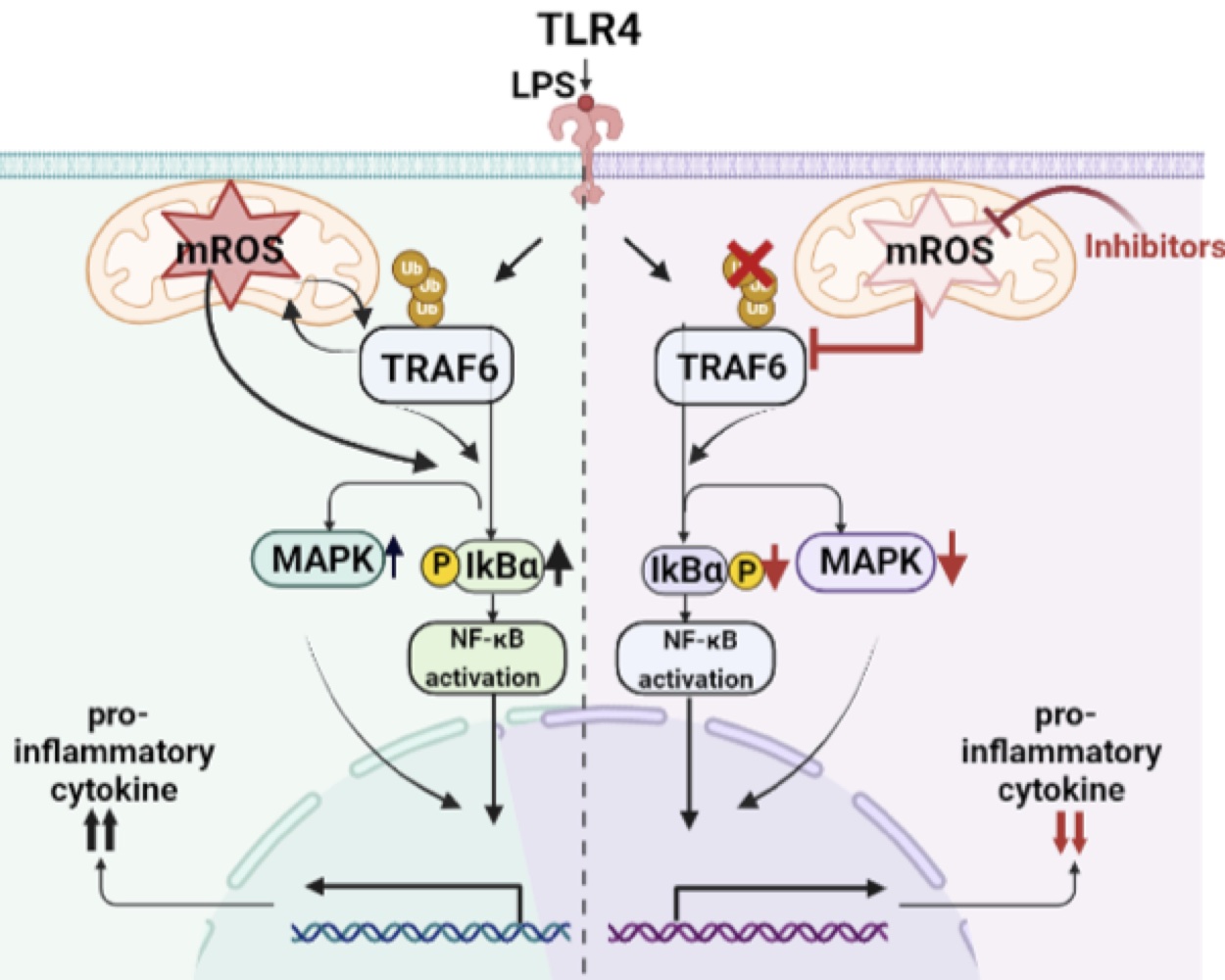
Impact of early mROS on the TLR4 pathway in stimulated macrophages. Our findings showed that early mROS signaling promoted NF-κB, MAPK activation as well as pro-inflammatory cytokine production, which was reduced by inhibition or quenching of mROS. Mechanistically, mROS could regulate TRAF-6 ubiquitination and initiate inflammatory responses and conversely TRAF-6 ubiquitination could induce mROS production.
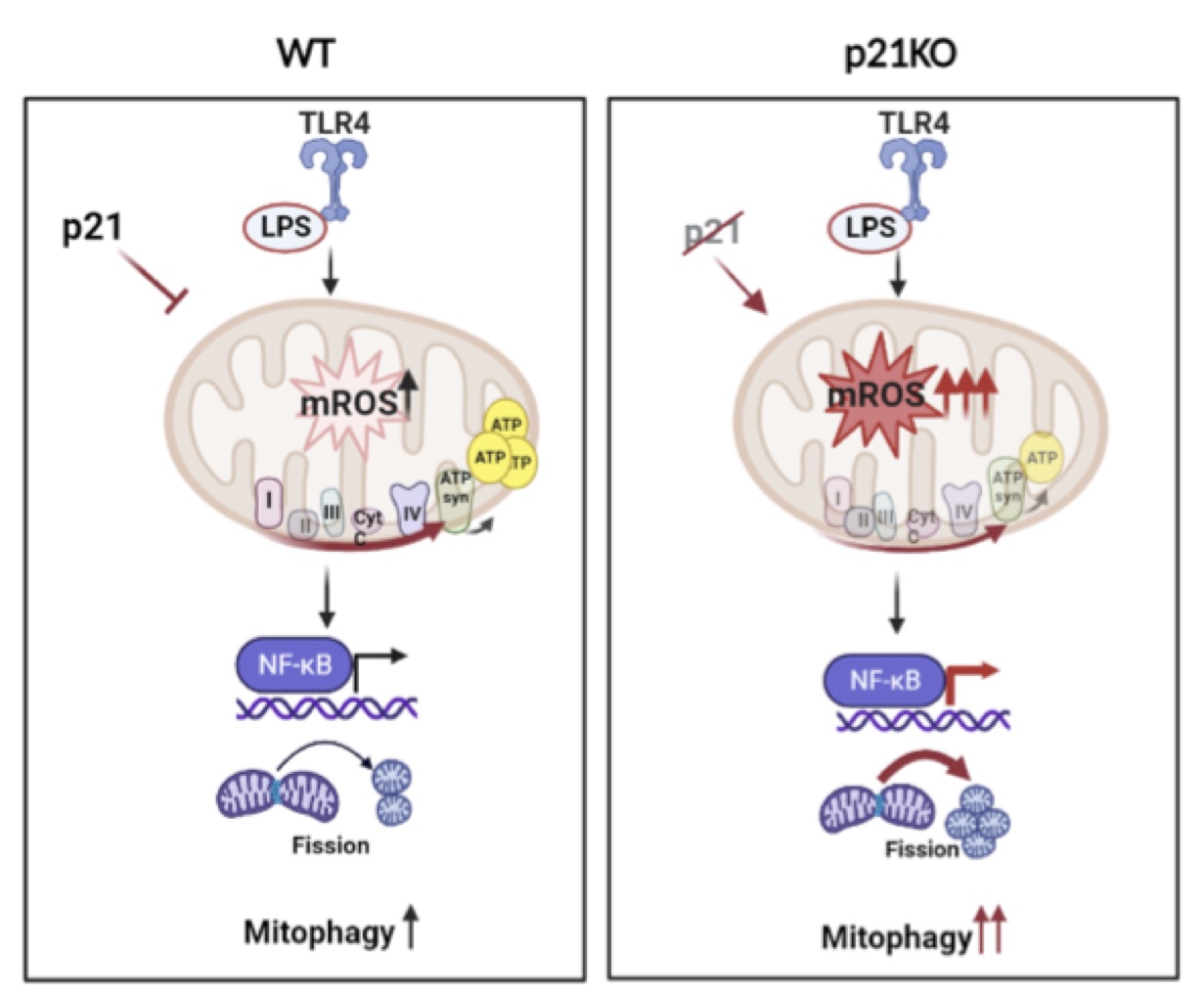
Macrophage responses are influenced by p21 and mROS. In the absence of p21, mROS levels, mitochondrial fission, mitophagy, and activation of NF-κB were increased.
Publications
Group Members
Group Leader
Dimitrios Balomenos
Funding
Our research is funded by national and international institutions as indicated below. For more details, please check the general Funding Section at the CNB website.

News
EMPLEO JOVEN 2025
Oferta: EMPJOV25_CNB_M2_A_034 El CNB-CSIC oferta una plaza de Técnico en gestión de proyectos I+D+i en la convocatoria del Programa de Empleo Juvenil del FSE+ 2025 (REF 38092) dirigida a jóvenes menores de 30 años, con título de grado de menos de 300 créditos ECT,...
Las Sinergias Severo Ochoa financiarán 8 proyectos colaborativos de grupos del CNB
La convocatoria interna Sinergias Severo Ochoa del Centro Nacional de Biotecnología (CNB-CSIC) financiará ocho proyectos colaborativos de grupos de investigación del centro, impulsando el desarrollo de programas transversales de alta calidad científica. Entre las...
Participamos en una jornada piloto de seguimiento de ayudas predoctorales de la Agencia Estatal de Investigación
El Centro Nacional de Biotecnología del Consejo Superior de Investigaciones Científicas (CNB-CSIC) es uno de los centros de investigación seleccionados para albergar jornadas piloto de seguimiento de las ayudas a la Formación del Personal Investigador (FPI) impulsada por la Agencia Estatal de Investigación (AEI).





