Nuclear Receptor Signaling
RESEARCH GROUPS

Mercedes Ricote
Group Leader
Research Summary
Our group investigates the transcriptional and epigenetic control of immune and cardiovascular cells in homeostasis and disease, with a particular focus on the molecular mechanisms underlying the actions of nuclear receptors. We use a combination of macrophage-specific knockouts, mouse models of disease, in vivo imaging, and next-generation sequencing technologies combined with bioinformatics approaches.
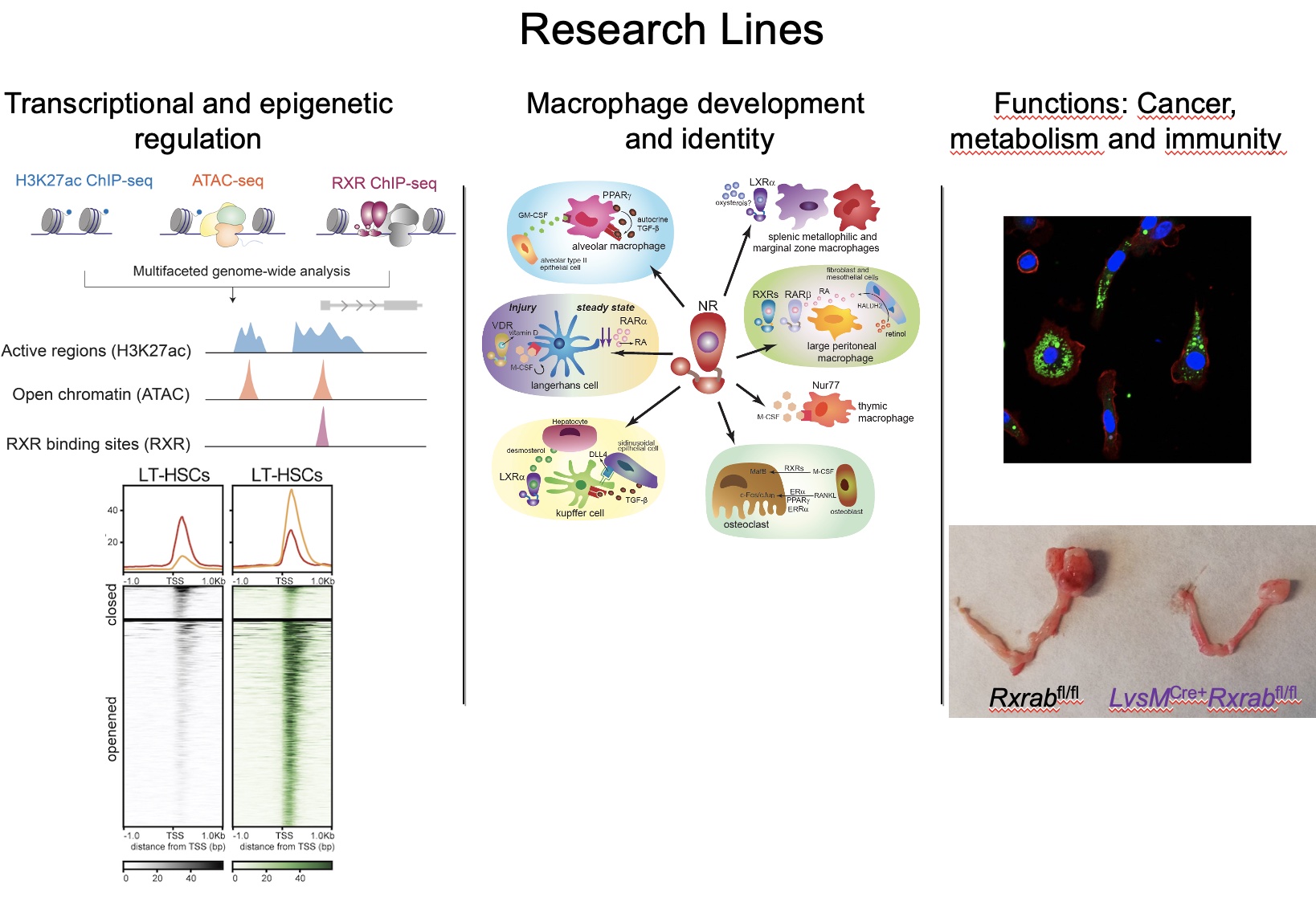
Research Lines
Different macrophage populations express distinct sets of transcription factors that are refined by tissue-specific microenvironmental cues. There is currently no clear understanding of the signaling molecules and mechanisms that drive the differentiation and specialized functions of most tissue-resident and monocyte-derived macrophages. Our group has made important contributions to deciphering the role of retinoid X receptor (RXR) nuclear receptor both in the generation of specialized tissue-resident macrophage subsets in homeostasis and in the actions of monocyte-derived macrophages in inflammation and tissue injury. Ongoing studies in the lab are exploring the role of RXR in macrophage identity and function and in the control of myeloid cell differentiation from hematopoietic stem cells.
A key focus of our current work is deciphering the transcriptional and epigenetic mechanisms controlling tissue macrophage identity, origin, and function homeostasis and defining how these mechanisms impact physiological and pathological responses in metabolic and cardiovascular diseases, and cancer. We recently identified novel factors and epigenetic regulators in the injured heart and uncovered the transcriptional variety of heart macrophages after myocardial infarction. This work revealed the presence in the heart of distinct macrophage subsets with different reparative capacities, and we are currently exploring the roles of these factors and macrophage populations and how macrophages sense local environmental cues during cardiac repair.
Other work in the laboratory is aimed at elucidating how nuclear receptors control cardiomyocyte physiology. Cardiomyocytes are thought to be functionally, and transcriptionally stable cells fully committed to contractile function. However, recent evidence suggests that the maintenance of cardiac function in health and disease depends on epigenetic regulation. We are particularly interested in the impact of RXR on chromatin remodeling and epigenetics in the heart during development, adulthood, and disease. In addition, we have recently initiated a new research direction examining the transmission of signals between mothers and neonates and their impact on the control of energy homeostasis.
Publications
Paredes A, Justo-Méndez R, Jiménez-Blasco D, Núñez V, Calero I, Villalba-Orero M, Alegre-Martí A, Fischer T, Gradillas A, Sant’Anna VAR, Were F, Huang Z, Hernansanz-Agustín P, Contreras C, Martínez F, Camafeita E, Vázquez J, Ruiz-Cabello J, Area-Gómez E, Sánchez-Cabo F, Treuter E, Bolaños JP, Estébanez-Perpiñá E, Rupérez FJ, Barbas C, Enríquez JA, Ricote M. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature. Jun;618(7964):365-373, 2023.
Menéndez-Gutiérrez MP, Porcuna J, Nayak R, Paredes A, Niu H, Núñez V, Paranjpe A, Gómez MJ, Bhattacharjee A, Schnell DJ, Sánchez-Cabo F, Welch JS, Salomonis N, Cancelas JA. Ricote M. Retinoid X receptor promotes hematopoietic stem cell fitness and quiescence and preserves hematopoietic homeostasis. Blood. Feb 9;141 (6):592-608, 2023.
Di Martino O, Ferris MA, Hadwiger G, Sarkar S, Vu A, Menéndez-Gutiérrez MP, Ricote M, Welch JS. RXRA DT448/9PP generates a dominant active variant capable of inducing maturation in acute myeloid leukemia cells. Haematologica. Jun 17, 2021.
Alonso-Herranz L, Sahún-Español A, Gonzalo P, Gkontra P, Núñez V, Cedenilla M, Villalba-Orero M, Inserte J, Clemente C, García-Dorado D, Arroyo AG, Ricote M. Macrophages promote endothelial-to-mesenchymal transition via MT1MMP/TGFβ1 after myocardial infarction. Elife. Oct 16;9: e57920, 2020.
Casanova-Acebes M, Menéndez-Gutiérrez MP, Porcuna J, Álvarez-Errico D, Lavin Y, García A, Kobayashi S, Le Berichel J, Núñez V, Were F, Jiménez-Carretero D, Sánchez-Cabo F, Merad M, Ricote M. RXRs control serous macrophage neonatal expansion and identity and contribure to ovarian cancer progression. Nat Commun. Apr 3;11(1):1655, 2020.
Group Members
Group Leader
Mercedes Ricote
Lab asistant
Patricia Cozar López
PhD Candidates
Laura Casablanca Osorio

News
PhD for the “Nuclear Receptor Signaling” group
IP: Mercedes Ricote En el grupo buscamos candidato/a para solicitar las convocatorias abiertas actualmente: Predoctorales-CAM-2024, COMUNIDAD DE MADRID y Ayudas para la formación de profesorado universitario (FPU) 2024, MINISTERIO DE CIENCIA, INNOVACIÓN Y...
Mercedes Ricote, XX Premio Ciencias de la Salud Fundación Caja Rural Granada por su trabajo sobre el papel de la leche materna en la maduración del corazón
23 de Julio 2024 Esta investigación, publicada en Nature, describe el mecanismo por el que un ácido graso presente en la leche materna regula la maduración de las células cardiacas tras el nacimiento Los resultados podrían tener implicaciones terapéuticas en algunas...
Funding
Our research is funded by national and international institutions as indicated below. For more details, please check the general Funding Section at the CNB website.






