Novel Modes of Intercellular Communication
RESEARCH GROUPS

Rubén García Martín
Group Leader
Research Summary
The main goal of our lab is to shed light on the fundaments of novel means of intercellular and interorgan crosstalk, including extracellular vesicles, lipoproteins and protein complexes in living organisms. We aim to explore their participation in the regulation of physiological processes with a special focus on metabolism and how their dysregulation could drive the development of metabolic disorders, cardiovascular diseases, cancer and immune system-related pathologies.
Research Lines
Intercellular communication is essential for the coordination of complex processes in multicellular organisms. We have recently witnessed the discovery of unexpected mediators of cellular communication including extracellular vesicles, lipoproteins and protein complexes (Fig.1). Material transported by these carriers is delivered to nearby and distant cells, mediating important changes in cellular function. Cargo include coding and non-coding RNAs, proteins, lipids, DNA or even subcellular organelles such as mitochondria. This has vastly expanded the complexity of intertissue communication beyond our classical understanding and, at the same time, opened a fascinating new era for our comprehension on how cells talk to each other.
Figure 1: New modes of intercellular communication among nearby and distant cells (located in other organs), including extracellular vesicles (exosomes and microvesicles), lipoproteins and protein complexes. (Extracted from Brandao et al, J of Physiol 2022, 600.5 pp1155-1169).
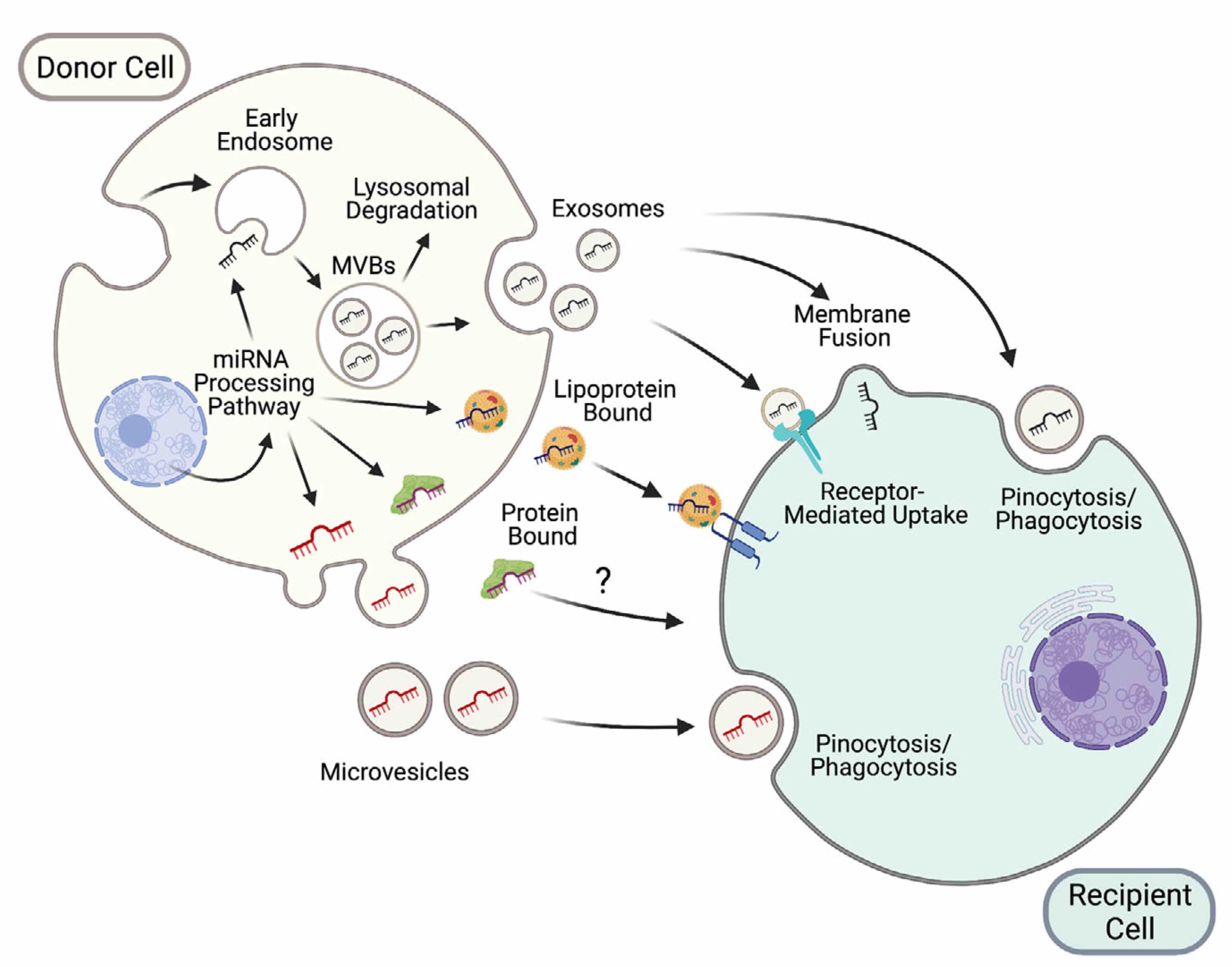
We have recently contributed to this exciting and unexplored area of research. For instance, we recently elucidated the mechanisms that guide the sorting of one of the major constituents of these carriers, microRNAs, in extracellular vesicles (Garcia-Martin et al, Nature 2022). We found that this process is largely mediated by the presence of short sequences located towards the 3’end of microRNAs that ultimately determine whether the harboring miRNA must be loaded into extracellular vesicles (by EXOmotifs) or retained in the producing cell (by CELLmotifs) (Fig. 2).
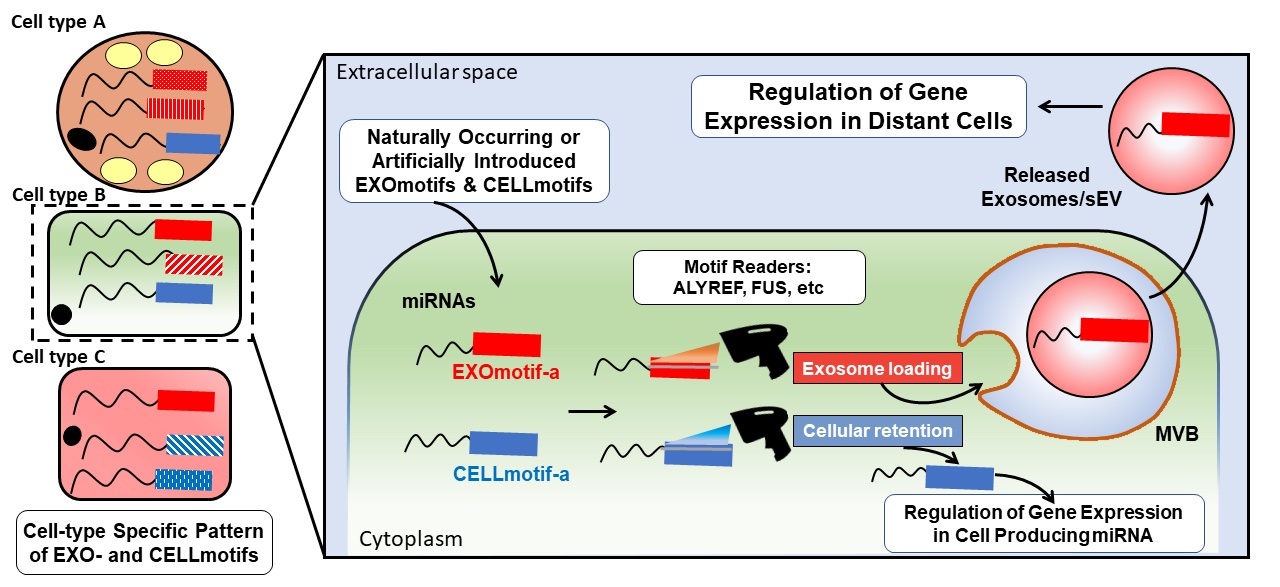
Each cell type has a specific pattern of EXO- and CELL-motifs regulating exosomal loading and cellular retention of microRNAs, respectively. Some proteins such as ALYREF and FUS are able to read those codes and mediate vesicular loading (Extracted from Garcia-Martin et al, Nature 2022, 601:446-451).
Besides microRNAs, we recently profiled the other major subset of extracellular vesicle cargo, i.e. proteins, and found that each major metabolic cell type releases a unique signature of proteins (mostly metabolism-related) into its extracellular vesicles (Garcia-Martin et al, Cell Reports, 2022). These proteins would reach target cells, get incorporated into the metabolic machinery and thereby influence the ability of those target cells to carry out different metabolic pathways. Recent exciting data, including ours, show that those microRNAs and proteins just represent a snapshot of the cargo released in vesicles under basal conditions. In fact, the content of extracellular vesicles, and thus the messages that cells send, constitute a dynamic process, which is thought to be tightly regulated by the cell’s exposure to a variety of factors. Along this line, we recently found that metabolic mediators such as insulin, the main hormone regulating glucose levels, induce substantial changes in the pattern of microRNAs secreted into extracellular vesicles (Lino, Garcia-Martin et al, Cell Reports 2024). Under high insulin conditions, these vesicles would likely act as “metabolic synchronizers”: they would arrive and deliver their new cargo to target cells to adapt their metabolic pathways to the new nutritional status, making these cells better able to cope with the high glucose and lipids that are characteristic of postprandial states.
Despite these and other fascinating recent findings, most key aspects of these communication systems remain largely unknown, especially in vivo. Our laboratory is currently developing innovative tools to better understand how extracellular vesicles and other carriers work in living organisms, both in health and disease. It is noteworthy that these novel cargo carriers have been shown to contribute in as yet unknown ways to numerous pathologies, including metabolic diseases, cancer, autoimmune disorders, cardiovascular diseases. In this context, we have recently shown that the content of extracellular vesicles participates in the development of lipodystrophy in HIV patients (Srinivasa, Garcia-Martin et al, JCI Insight 2021). Thus, a better understanding of the biology of these novel communication systems could lead not only to an unprecedented expansion of the list of molecular agents that mediate our cellular dialogs, but also to innovative diagnostic and therapeutic applications for many pathologies.
Publications
Lino M, Garcia-Martin R, Rosetto-Muñoz V, Palermo Ruiz G, Nawaz A, Brandão BB, Dreyfuss J, Pan H, and Kahn CR. Multistep Regulation of MicroRNA Expression and Exosomal Secretion by Insulin. Cell Reports 2024 Jul 23;43(7):114491.
Garcia-Martin R, Wang G, Brandao BB, Zanotto TM, Shah S, Patel SK, Schilling B, Kahn CR. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022 601(7893):446-451
Garcia-Martin R, Brandao BB, Thomou T, Altindis E, Kahn CR. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Reports 2022, 38(3):110277.
Srinivasa S, Garcia-Martin R, Torriani MD, Fitch KV, Carlson A, Kahn CR, Grinspoon SK. Altered Pattern of Circulating miRNAs in HIV Lipodystrophy Perturb Key Adipose Differentiation and Inflammation Pathways. JCI Insight 2021, 6(18):e150399. doi: 10.1172/jci.insight.150399.
Mori M, Ludwig R, Garcia-Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: From Biomarkers to Mediators of Homeostasis and Disease. Cell Metabolism, August 2019. 30(4):656-673. doi: 10.1016/j.cmet.2019.07.011.
Group Members
Group Leader
Rubén García Martín
Staff Scientist
Ángela Rey Gallardo
PhD candidates
Paula Díez Roda

News
Técnico/a de laboratorio – Nuevas formas de comunicación intercelular
IP: Rubén García-Martín Para desarrollar nuestras líneas de investigación, buscamos un técnico de laboratorio para llevar a cabo soporte experimental en el laboratorio, así como realizar tareas de organización interna del laboratorio. Entre las tareas a realizar...
Looking for a postdoc in the “Novel means of intertissue communication” lab
IP: Rubén García Martín We are looking for a postdoctoral candidate to apply for a Juan de la Cierva (Spanish State Research Agency, AEI; please note tight deadline below) fellowship based on the following criteria: PhD in a topic related to Metabolism and/or...
PhD position in “Novel means of intertissue communication” lab
IP: Rubén García Martín Buscamos candidato/a para empezar el doctorado con las siguientes cualidades: Máster en Biotecnología, Bioquímica, Biomedicina o similar, o que espere terminarlo en el curso académico 2024/2025. Alta motivación por realizar una carrera...
Funding
Our research is funded by national and international institutions as indicated below. For more details, please check the general Funding Section at the CNB website.




