Innate Immunity to Viruses and Cancer
RESEARCH GROUPS

Hugh Reyburn
Group Leader
Research Summary
Studies of the cell biology of cytotoxic lymphocytes in cancer and virus infections, particularly those associated with herpes viruses, is the central focus of our research. These viruses establish persistent infections and are important causes of morbidity and mortality in patients with depressed immune system function. My group studies the immune responses to these viruses, as well as how these viruses try to evade immune surveillance. We also collaborate intensively with clinical colleagues in studies of these infections in patients with genetic mutations that impair immune system function as a source of in vivo insights into the effects of defective innate immunity on disease pathogenesis in these individuals. Once the genetic defect has been identified, and the phenotypic and functional defects have been characterised in detail we study the cell biology of specific molecules to try and achieve a mechanistic explanation of the genotype-phenotype relationship observed in vivo. In this work we collaborate with a network of national and international basic scientists and clinicians.
Research Lines
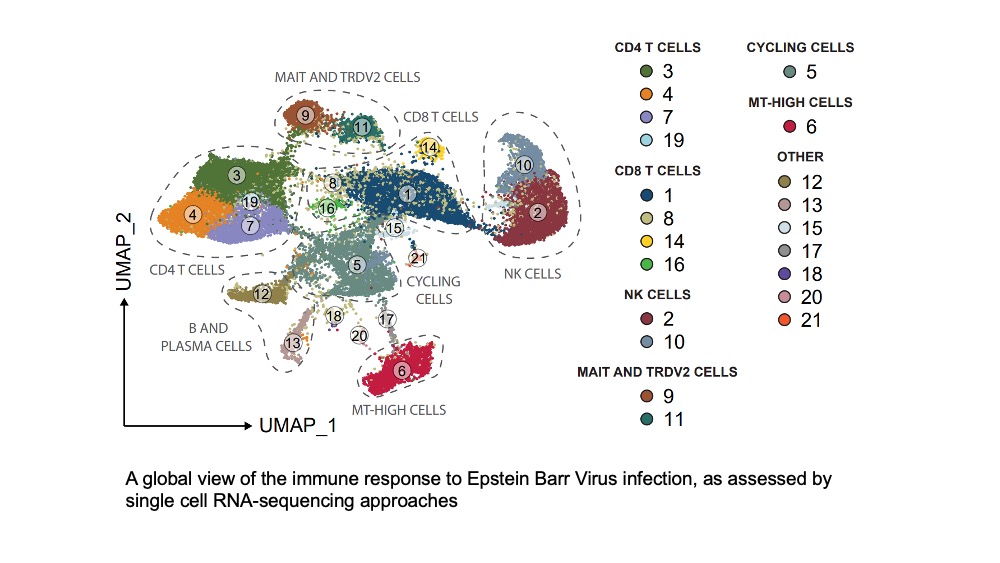
The innate immune system is the frontline defense of the human body and allows for rapid recognition of an invading viral pathogen or developing tumour. Natural killer (NK) cells are the lymphocytes of innate immunity and can kill infected cells, and secrete cytokines, to play an important role in immune responses to viral infection and cancer. Although NK cells are often perceived as rather primitive lymphocytes; always ready to kill unless checked by inhibitory receptors binding to MHC Class I molecules. It is now clear that the behaviour of an NK cell when confronted by a potential target cell depends on the integration of multiple signals coming from a range of activating and inhibitory receptors. Inhibitory receptor expression is largely under genetic control, whereas activation receptor expression is heavily environmentally influenced and NK cells adapt their expression of activating receptors in response to pathogens and tumours so giving rise to the multiple discrete NK cell subpopulations that can be found in human peripheral blood. Thus, to understand NK cells in disease requires detailed knowledge of the biochemistry of individual activating and inhibitory receptors and the subpopulations of NK cells expressing different receptor repertoires.
In the past, we have contributed extensively to knowledge of the biochemistry and cell biology of various NK cell receptors and their ligands. To address the wider roles of NK cells in immune defense, we collaborate with clinical colleagues to study patients suffering from genetic mutations that affect NK cell function. Inherited human immunodeficiencies are experiments of nature in which gene defects compromise immune function and our hypothesis is that the study of congenital defects affecting NK cells will help to increase our understanding of NK cell biology and function in vivo.
More recently, during the COVID-19 pandemic we developed assays to analyse the antibody response to SARS-CoV-2 infection (subsequently licenced to the World Health Organisation, for use in less developed countries) and have gone on to study the interactions between antibodies and Natural Killer cells in immunity to SARS-CoV-2 in elderly people.
Nearly all of our science is carried out using innovative flow cytometry and molecular genetic technologies as well as classical cellular and biochemical approaches. These studies are complemented and enhanced by in vitro experiments involving the use of genome-editing techniques to study in detail the molecular bases of the changes observed in vivo. Since we work only with human samples ex-vivo these experimental approaches allow us to obtain high resolution analyses of human immune function in real-world conditions.
Publications
Group Members
Group Leader
Hugh Reyburn
Lab assistant
Ruth Gómez-Caro Gil
Postdoctoral Researcher
Álvaro Fernando García Jiménez
PhD candidate
Andrea Sánchez de la Cruz





