Diacylglycerol Kinases and Immune Functions
RESEARCH GROUPS

Isabel Mérida
Group Leader
Research Summary
The emergence of immunotherapies in the recent years has shifted the paradigm of cancer therapies, directing them to rescue the ability of T lymphocytes to eliminate tumors. Our group works to demonstrate that the fine-tuning of Diacylglycerol-based signaling by Diacylglycerol Kinases (DGKs) represents a critical aspect of T cell functions. Studies in human and mice have consistently demonstrated that targeting specific DGK isoforms enhances the ability of both natural and engineered T lymphocytes to eliminate tumors. However, the exact contribution of each independent isoform as well as the consequences of their pharmacological targeting have not been fully explored. Which DGK isoform is the best target, what type of cancers will be sensitive to DGK modification, how DGK inhibitors will cooperate with other anticancer therapies and the identity of biomarkers to better stratify patients are questions that unsolved. Another important issue is the potential undesired unleashing of autoimmune reactions as the result of DGK inhibition. Our team uses different approaches to fully understand the potential of DGK targeting as a novel mechanism to fight cancer.
Research Lines
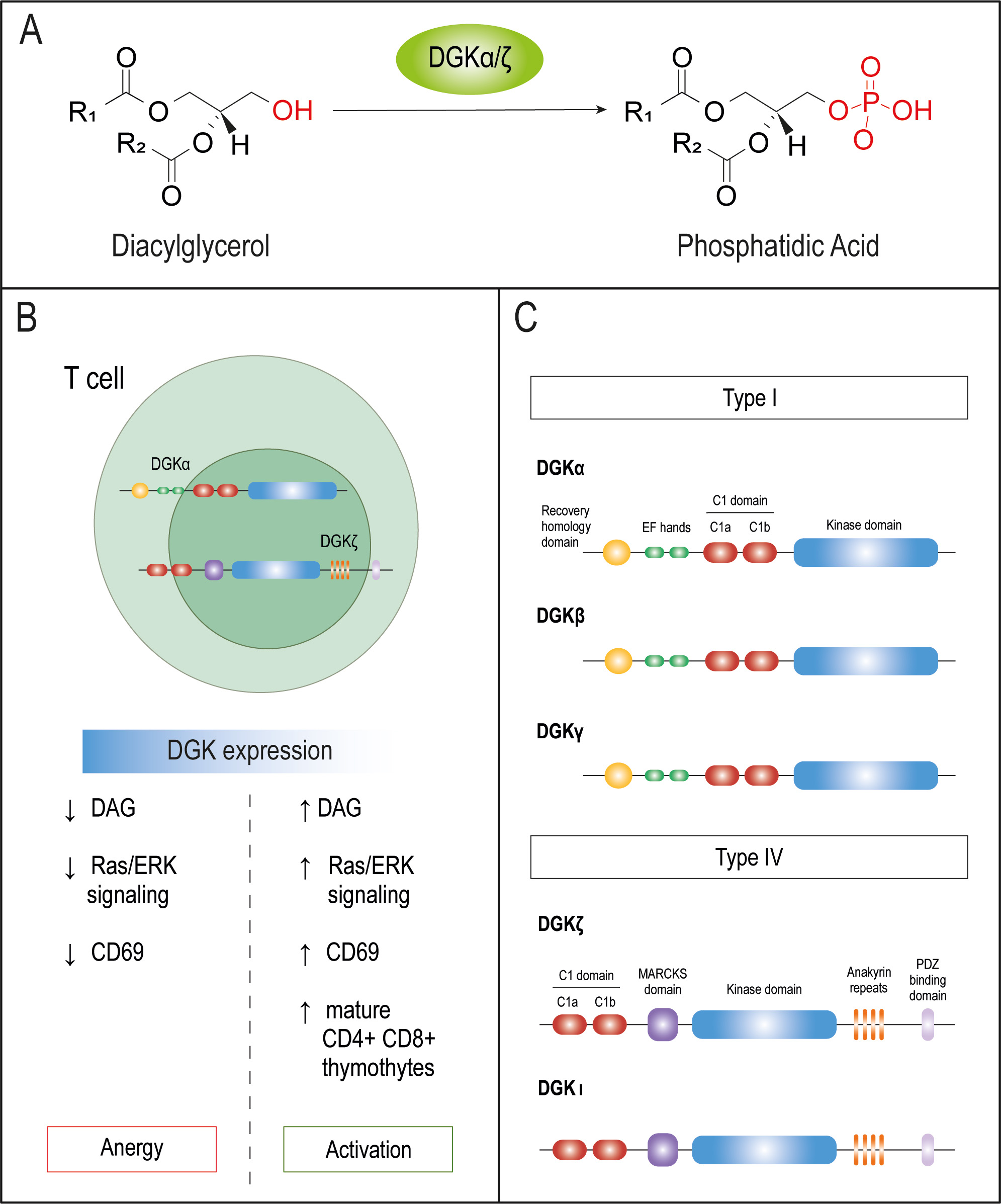
DGK dependent control of T cell activation. Diacylglycerol Kinases are an ample family of enzymes that catalyze the phosphorylation of Diacylglycerol (DAG) into phosphatidic acid (Fig 1A). Along the years our studies have contributed to demonstrate that, although not an immune checkpoint in the classical sense, the DGKa and DGKz isoforms operate as potent physiological rheostats of T cell functions. We use different approaches to assess their redundant and specialized functions, their mechanisms of regulation and the identity of its partners.
DGK activity facilitates tumor immune evasion and metastasis. The abnormal upregulation of DGKa and z both in natural T lymphocytes and engineered CAR-T cells infiltrating solid tumors facilitates the acquisition of anergic/exhausted phenotypes that facilitate tumor immune evasion (Fig 1B). Pharmacological DGK targeting was, until very recently, a theoretical concept due to the lack of isoform-specific inhibitors. In the recent years we have initiated new and fruitful collaboration with pharmaceutical companies to help in the characterization of DGK inhibitors amenable for clinical use.
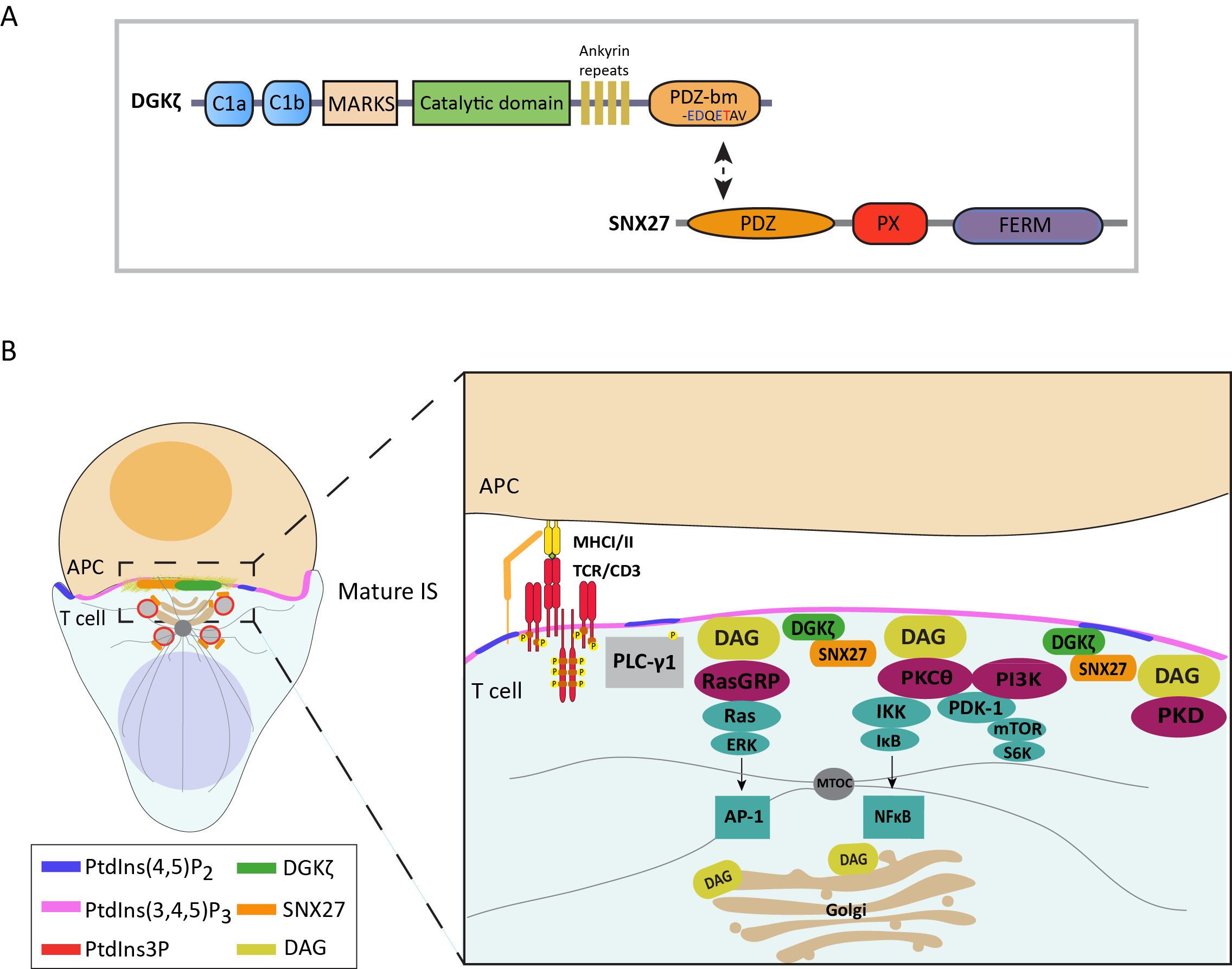
Sorting Nexin 27 provides spatial regulation of DGK function. One of the main differences between type I DGKa and type IV DGKz resides at their C-terminus where DGKz contains a C-terminal class 1 PDZ-binding motif (PDZ-bm) (Fig 1C). Using proteomic approaches, we identified Sorting Nexin 27 (SNX27) as a PDZ dependent interactor of DGKz. SNX27 interaction with DGKz provides adequate control of DAG consumption during antigen recognition and helps to sustain MTOC polarization during immune synapse formation (Fig 2). Decreased SNX27 expression in the brain has been related with synaptic dysfunction and is associated with Trisomy 21 (T21) and Alzheimer disease (AD), both heavily linked to neuroinflammation. To investigate the contribution of SNX27 to inflammatory mechanisms we have generated mice deficient for SNX27 either in the T or myeloid lineage. These models suggest that SNX27 deficit provides a chronic inflammatory milieu characteristic reminiscent to that described in Down Syndrome.
DGK-dependent control of the innate response. DGK functions in the myeloid subsets have been poorly studied, particularly in human cells. In the last years and as the result of our research in COVID-related areas, we have initiated new studies aimed to investigate the contribution of DGKa and DGKz in the regulation of innate-related responses.
Publications
Group Members
Group Leader
Isabel Mérida
Project Leader
Juana Antonia Avila-Flores
Postdoctoral Scientist
Elena Andrada
PhD candidates
Miguel Martin Salgado
Juan José Sánchez Cabezón
Ane Ochoa Echeverria
Lab manager
Rosa Mª Liébana Gallego
Lab assistant
Ana Isabel Checa

Funding
News
Identifican el papel de la proteína SNX27 en las alteraciones inmunológicas asociadas al síndrome de Down
15 enero 204 La SNX27 es una proteína que regula el tráfico de proteínas y su deficiencia afecta las funciones cognitivas en humanos y en modelos de ratón. En el síndrome de Down se observa una deficiencia en los niveles de SNX27 que se ha asociado con defectos...




