Antibody-Based Agents for Cancer Immunotherapy
RESEARCH GROUPS

Leonor Kremer
Group Leader
Research Summary
Immunotherapy, a clinical treatment that modulates the immune system to cure different diseases, has provided excellent results in cancer fighting in the last years. However, new drugs are needed to improve and broaden the application of these therapies. The main goal of our laboratory is to generate and characterize new monoclonal antibodies, directed against membrane antigens of tumor cells, with high potential as therapeutic agents for cancer treatment. We are currently in the advanced stages of developing an antibody directed against the chemokine receptor CCR9, which is over-expressed in the tumor cells of the vast majority of patients with T-cell acute lymphoblastic leukemia (T-ALL). We have also generated a panel of antibodies against other tumor cell surface targets for use in immunotherapy cocktails.
Research Lines
For decades, our group has studied the role of chemokines and their receptors in tumor progression and metastasis, focusing on assessing the potential of chemokine receptors as possible anti-tumor targets. In this context, we have generated a panel of mouse monoclonal antibodies (mAbs) specific for the human chemokine receptor 9 (CCR9), a protein overexpressed in different types of hematological neoplasias. CCR9 is expressed in the malignant cells of around 80% of patients with T-cell acute lymphoblastic leukemia (T-ALL), a severe disease that mainly affects children and young adults. Despite recent advances in their treatment, a high percentage of these patients are non-responders or suffer relapses.
Two of the anti-CCR9 mAbs we have developed, 91R and 92R, have been selected for their high efficacy in reducing the growth of human CCR9+ tumors in several xenogeneic mouse models and have been protected by an international CSIC patent licensed to SunRock Biopharma. Chimeric and humanized variants have been generated from these antibodies that also effectively inhibit tumor progression. However, the 91R and 92R mAbs do not recognize rodent CCR9, which would require the use of a large number of primates in order to perform the required regulatory preclinical assays. To circumvent this problem, we have recently generated a genetically modified mouse which expresses a partially humanized CCR9 receptor and thus allows the study of tumor growth inhibition, off-target effects, toxicity and biodistribution of anti-CCR9 mAbs in syngeneic immunocompetent mouse models.
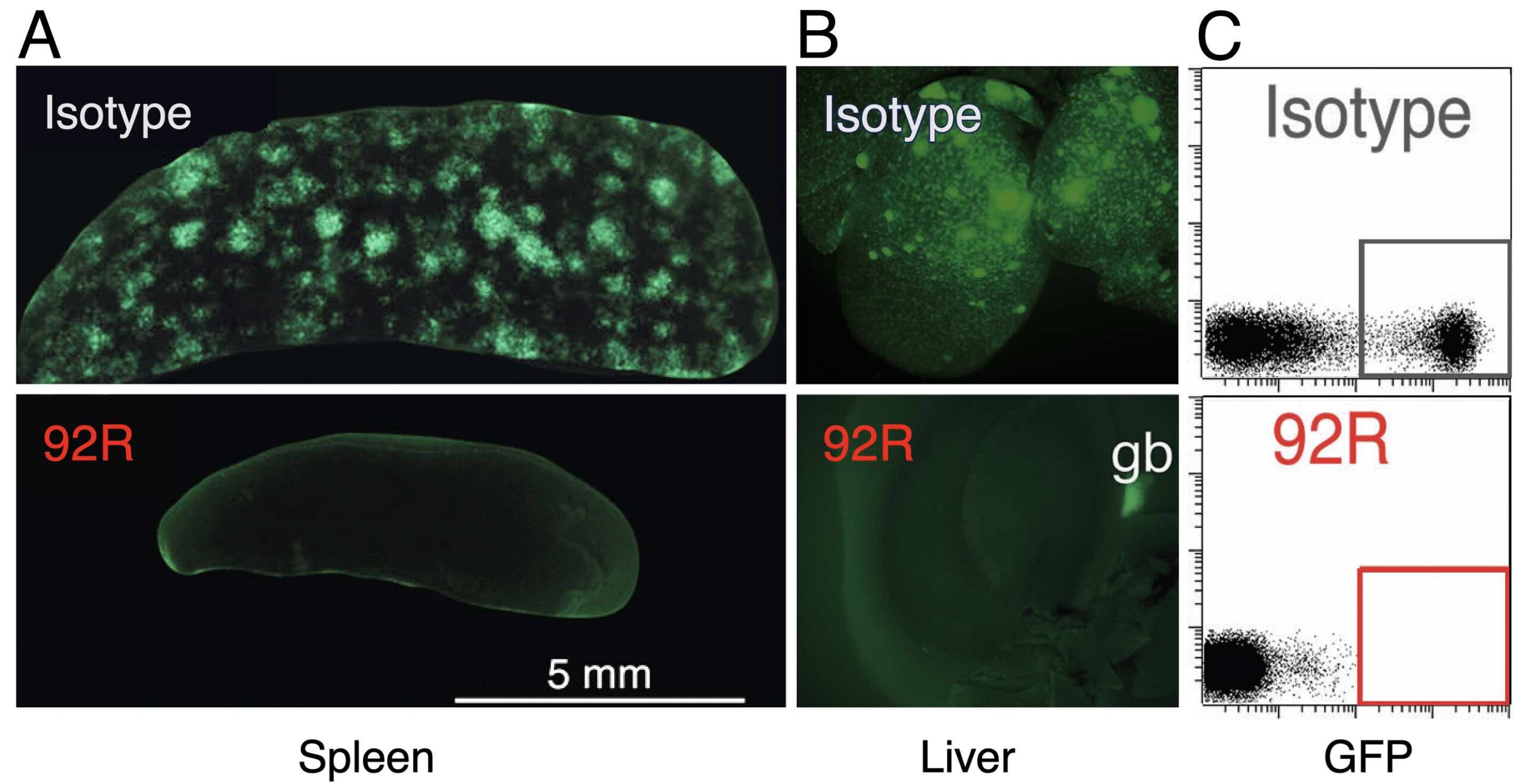
Effect of 92R mAb on the progression of MOLT4-GFP orthotopic xenotransplants. Tumor cells were injected in the tail vein of NSG mice, which were separated into two groups and treated with either 92R mAb or with an isotype control mAb. (A) Representative stereomicroscopic images of spleens (A) and livers (B) from mice of each group. Representative flow cytometry analyses showing the tumor cells (the fraction of GFP+ cells) in the bone marrow on isotype control– and 92R mAb-treated animals (C).
Therapies against a single target on the tumor cell are often ineffective, due to the proliferation of tumor cell populations that are resistant to the therapeutic agent used. Therefore, to increase the chances of success of anti-CCR9 antibody therapy, we have recently generated mAbs against other human T-ALL cell surface antigens, using whole tumor cells as immunogens. These mAbs could be used as antibody-drug conjugates or as part of antibody cocktails capable of synergistically attacking different molecular targets on tumor cells. Among them, we have selected several mAbs that strongly reduce tumor size in orthotopic animal models. Using proteomic techniques and protein arrays, we have identified the cellular antigens recognized by those mAbs that have demonstrated a higher therapeutic potential in in vivo assays, together with high specificity and a subnanomolar affinity level for their respective target antigens.
In the context of a scientific collaboration within the CNB NanoBioMedicine Initiative, we are developing mAbs that target nanoparticles to specific types of tumor cells, and the 92R mAb has been used to functionalize magnetic nanoparticles that specifically recognize human CCR9+ cells for use in magnetic hyperthermia therapy. In other collaborations, we are also generating and evaluating mAbs that could be used to modulate the immune response in other pathologies. We have contributed to analyze the role of the CCL1-CCR8 axis in atherosclerosis; to study CD5L in liver fibrosis and cancer; to generate new tools to inhibit viral uptake of Ebola and HIV-1; and to generate and characterize mAbs against SARS-CoV-2, which are being assessed as potential therapeutic agents for the treatment of COVID-19.
Inhibition by 92R mAb of progression of primary human T-cell leukemia HLPR (CCR9+) cells in immunodeficient mice. Flow cytometry analysis of CCR9 expression on HLPR cells (A). Mouse survival experiments (B), 1×106 HLPR cells were injected into the tail vein of NSG mice. Animals were treated, on days 2, 9 and 85, with 4 mg/kg/dose of 92R mAb (red) or isotype control mAb (IgG2a, grey).









