Structural Biology of Virus Proteins
RESEARCH GROUPS

Mark van Raaij
Group Leader
Research Summary
Bacteriophages specifically recognize and kill host bacteria and they or their bacteriolytic enzymes are emerging as an alternative infection treatments. Detailed knowledge of how bacteriophage receptor binding proteins interact with bacterial membrane components will be of great help to develop new applications. At the end of their infection cycle, bacteriophages produce specific endolysins designed to cleave host peptidoglycan and release their progeny. We study the structures of both types of proteins in complexes with carbohydrate fragments of the cell wall. A separate research line is the structural biology of adenovirus proteins. Here we are especially interested in the fibre protein, which for most adenoviruses is the primary host cell recognition factor.
Research Lines
Bacteriophages are macromolecular machines that specifically recognize host bacteria, transfer their genetic material to them, and redirect the host cell machinery to produce more viral particles. Shortly after their discovery, phages began to be used as a treatment for bacterial infections, but after the discovery of antibiotics, they were relegated to the background. Currently, with the emergence of many bacteria resistant to antibiotics, phages and their bacteriolytic enzymes are emerging as an alternative in the treatment of various infections. A detailed knowledge of the infection mechanism of bacteriophages would be of great help to develop new applications. Potentially, by making mutations in the proteins they use to recognize bacterial receptors, the range of bacteria that a particular bacteriophage is able to recognize could be altered.
At the end of their infection cycle, bacteriophages produce specific endolysins designed to cleave host peptidoglycans and thus release their progeny. These proteins can be used for the eradication of pathogenic bacteria, but also for their detection. Endolysins can contain a single catalytic domain or multiple domains, including a cell wall binding domain. To understand their function in detail and design mutated or chimeric molecules with novel properties, it is necessary to know their structures and interaction mechanisms in detail.
Both receptor biding proteins and endolysins interact with the bacterial cell wall. We study the structures of both types of proteins in complexes with carbohydrate fragments of the cell wall and with complete bacteriophage tails. Knowledge of the structures of phage receptor biding proteins and endolysins forming complexes with oligo-saccharide receptors will allow us to determine how they bind to bacterial cell walls at the level of amino acid detail.
A separate research line is the structural biology of adenovirus proteins. Here we are especially interested in the fibre protein, which for most adenoviruses is the primary host cell recognition factor.
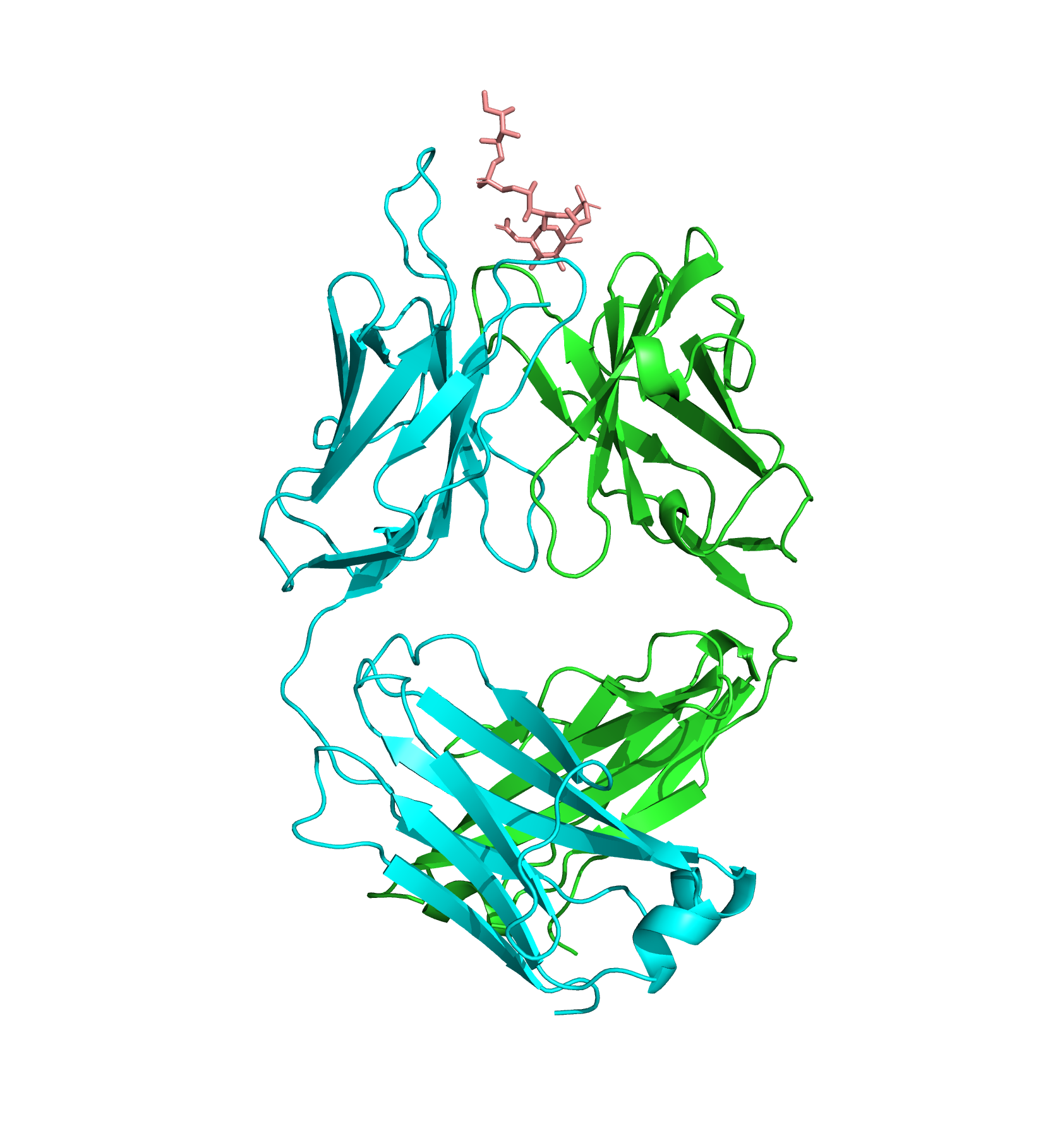
Structure of an Fab fragment of an antibody (blue and green) bound to teichoic acid (pink).
Publications
Group Members
Group Leader
Mark van Raaij
Postdoctoral Researchers
José Gallardo Hernanz
Pablo Herrera Nieto
(Long-term visitor)
Pablo Soriano Maldonado

Funding
News
PhD in the European Maria Sklodowska Network AUREUS
IP: Mark van Raaij A fully funded doctoral position in the area of Structural Biology is available at the Centro Nacional de Biotecnología (CNB-CSIC), Madrid, Spain (in collaboration with the Autonomous University of Madrid, where the degree will be registered). The...
iAds: Utilización de vectores de adenovirus para vacunas y transferencia de genes
20 de Febrero 2024 El proyecto iAds busca maximizar el potencial de los vectores de adenovirus para su uso en aplicaciones tales como las vacunas y la transferencia génica. Su objetivo persigue la búsqueda de soluciones en áreas médicas no cubiertas hasta ahora...






