Functional Analysis of Transcriptional Repressor DREAM
RESEARCH GROUP

José Ramón Naranjo
Group Leader
Research Summary
Our major research focus is on the multifunctional protein DREAM and its role in the changes in calcium and protein homeostasis associated with several neurodegenerative disorders including Huntington disease (HD), Alzheimer disease (AD) and Amyotrophic Lateral Sclerosis (ALS).
Research Lines
1.- Neuroprotective role of DREAM silencing in neurodegeneration. We have shown that expression of DREAM is reduced i) in the striatum of R6/2 mice as well as in Hdh111/111 neurons, a mouse and cellular models for Huntington’s disease, respectively (J Clin Invest.126:627-38 (2016)) and ii) in cerebral cortex and hippocampus from J20 and Tg2576 mice, two mouse models of Alzheimer’s disease, and in transgenic mice over-expressing TDP-43, a mouse model of Fronto Temporal Dementia and Amyotrophic lateral sclerosis. The reduction in DREAM levels has been also observed in brain, CSF and blood samples from human patients with these pathologies (Patent P9312EP00). Induced haplo-deficiency for DREAM in R6/2 mice, after crossing with DREAM deficient mice, delays the onset and progression of motor dysfunction (J Clin. Invest.126:627-38 (2016)). The mechanism involves the interaction between DREAM and ATF6 and the activation of the UPR. Thus, early down regulation of DREAM level in neurons during the pre-symptomatic phase of HD might be part of a neuroprotective mechanism that could also be active in AD and FTD. Interestingly, these results suggest that DREAM could be a novel and wide spectrum target for therapeutic intervention in neurodegeneration and that molecules able to bind to DREAM and block its physiological functions could be candidates to treat neurodegenerative disease patients (Patent PCT-P6529PC00).
2.- Development of DREAM ligands: Ca2+ binding to functional EF-hands in the DREAM protein triggers a conformational change that prevents binding to DRE sites in the DNA and modifies its affinity for some interacting proteins. Importantly, changes in DREAM conformation are also induced upon binding of small molecules like arachidonic acid and some diaryl- and sulfonyl-urea derivatives that produce changes in the modulation of Kv4 channels by DREAM, thus changing this biological property of DREAM. We have recently reported the Ca2+-dependent binding of repaglinide to recombinant DREAM by affinity chromatography and surface plasmon resonance and showed a delay onset and symptoms amelioration by repaglinide in R6/2 mice (J Clin Invest.126:627-38 (2016)). In collaboration with chemists at the Medicinal Chemistry Institute (CSIC, Madrid) we are currently assessing series of diaryl- and sulfonyl-urea derivatives for their application to treat neurodegenerative diseases (Sci Rep. 9:7260 (2019)).
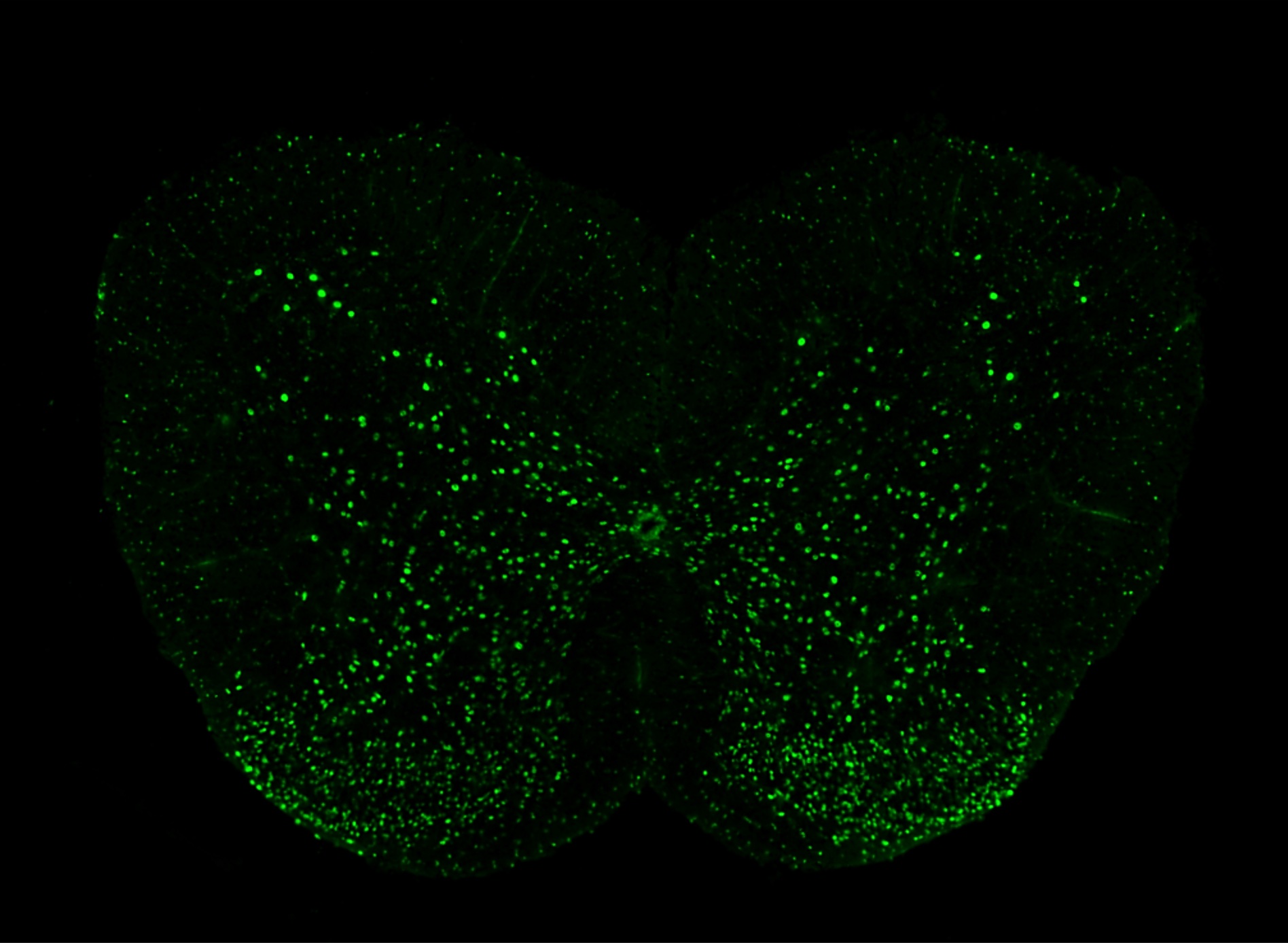
Lumbar spinal cord section showing TDP43 overexpressing neurons in a mouse model of Amyotrophic Lateral Sclerosis (ALS)
Publications
Group Members
Group Leader
José Ramón Naranjo
Postdoctoral Researcher
Rafael David Gonzalo Gobernado
Lab assistant
Xose Manuel Dopazo Santos
CIBERNED PERSONNEL
Lab assistant
Paz González Pérez



