Molecular Bases of Birnavirus Pathogenesis and Persistence
RESEARCH GROUPS

Dolores Rodríguez Aguirre
Group Leader

Fernando Almazán Toral
Group Leader
Research Summary
Our research focuses on understanding the interaction between the Birnavirus Infectious Bursal Disease Virus (IBDV) and host cells. Specifically, we aim to explore how the innate immune response influences the development and pathogenesis of IBDV infections, and to uncover the molecular mechanisms behind IBDV persistence. We are particularly interested in investigating the role of the interferon (IFN) pathway in IBDV-induced cell death through apoptosis, as well as the contribution of defective viral genomes (DVGs) to persistent IBDV infections. Additionally, in the context of the COVID-19 pandemic, we launched new research initiatives on SARS-CoV-2 aimed at developing innovative strategies to combat virus infection.
Research Lines
Infectious Bursal Disease Virus (IBDV) is an Avibirnabirus and the best characterized member of the Birnaviridae family. IBDV causes an acute, highly contagious, immunosuppressive disease, known as Infectious Bursal Disease (IBD) or Gumboro disease. This disease predominantly affects domestic chickens (Gallus gallus), leading to significant economic losses in the poultry industry worldwide. A number of studies have demonstrated that IBDV infection triggers an exacerbated expression of proinflammatory cytokines, including interferon (IFN). The infection leads to the loss of B lymphocytes through apoptosis and the destruction of the Bursa of Fabricius, the primary lymphoid organ in birds. This immunosuppression is a key factor in the severity of the disease in infected chickens. Several years ago, we initiated a collaboration with Dr. José F. Rodríguez (CNB) to explore the impact of the host’s innate immune response on the development and pathogenesis of IBDV infections. Our initial findings highlighted the crucial role of IFN in virus-induced cell death by triggering apoptosis in IBDV-infected cells, thereby exacerbating the disease in chickens. Additionally, our research has shown that IBDV can establish long-term persistent infections in cultured cells. Characterization of these persistently infected cell clones revealed their inability to respond to type I IFN, which explains their survival after IBDV infection. These findings underscore the significance of IFN in the outcome of IBDV infection. However, our understanding of the IFN system in birds remains limited compared to mammals. Consequently, we are also focused on investigating the regulation of the signaling pathway in chicken cells upon recognition of the double-stranded RNA (dsRNA) IBDV genome. Our research uncovered the regulatory role of TRIM25 in the MDA5 signaling pathway in chicken cells and its contribution to controlling IBDV infection.
On the other hand, as mentioned above, we have determined that IBDV can establish long-term persistent infections in cultured cells. In this sense, we are interested in deciphering the molecular bases of this persistency. Preliminary RNA-seq data from infected cells strongly suggest that IBDV downregulation is due to the accumulation of defective viral genomes (DVG) harboring large deletions. Hence, we aim at characterizing these DVGs, as well as their potential role in activating a cell survival program, similar to what has been observed in other virus-cell systems. Furthermore, a remarkable characteristic of persistently infected cells is their resilience to reinfection with IBDV. This phenomenon is likely attributable to the inhibitory effect of DVGs on IBDV replication. Consequently, our final objective is to investigate the potential of leveraging the inhibitory capacity of DVGs to generate IBDV-resistant cells through intracellular production of DVGs, thereby mimicking the resistance to superinfection observed in IBDV-persistently infected cells. Through these studies, we aim to deepen our understanding of the molecular mechanisms governing IBDV infections and enhance strategies to combat this virus, ultimately aiding in the protection of the poultry industry.
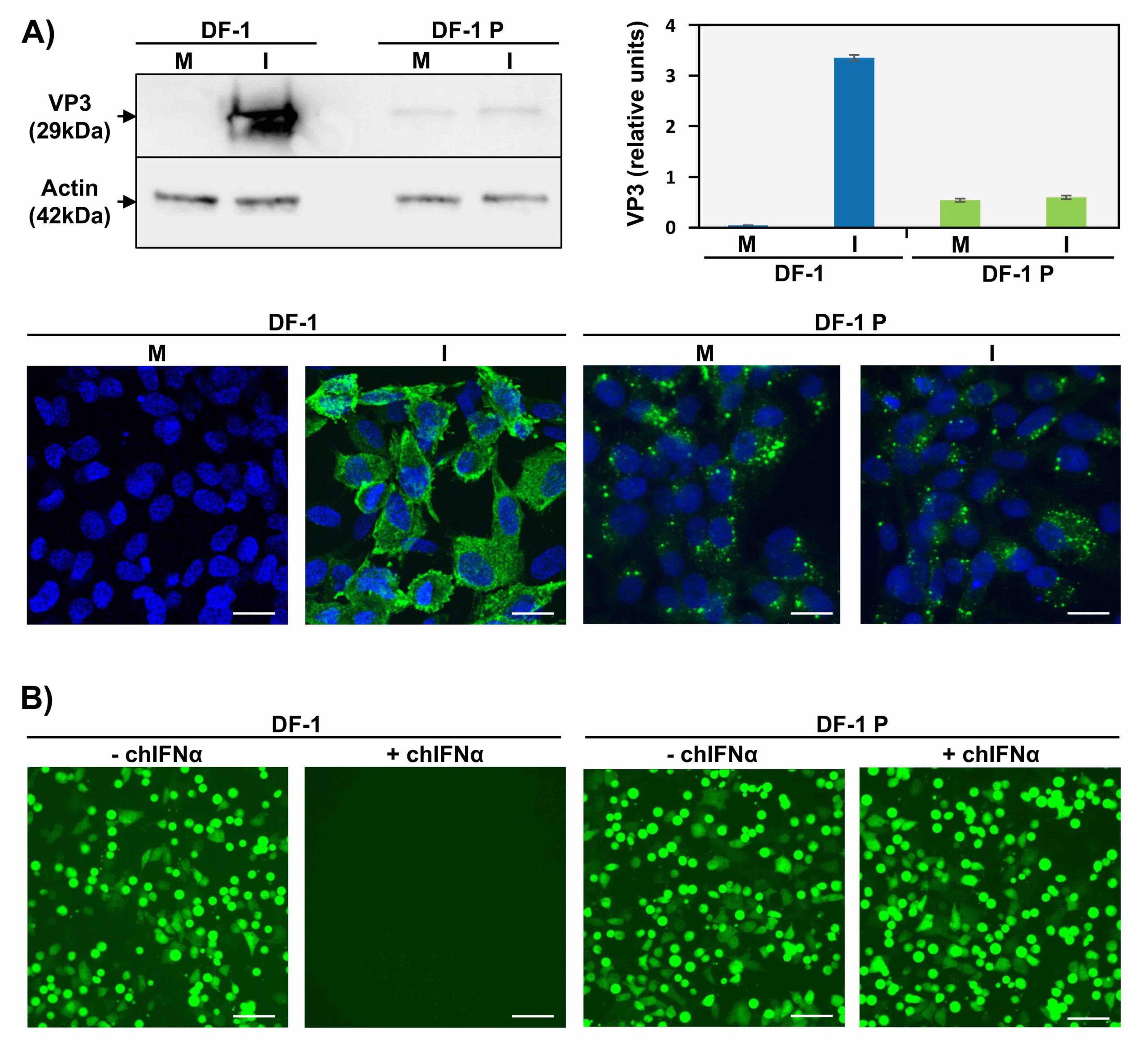
Characterization of IBDV persistence in DF-1 cells. A) IBDV replication in persistently infected cells. DF-1 or DF-1P (persistently infected) cells were mock infected (M) or infected (I) with IBDV and the expression of the viral protein VP3 was analyzed by Western-Blot (upper pannels) and immunofluorescence (lower pannels). DF-1P cells showed both low viral replication and resistance to IBDV reinfection. Scale bars, 20 µm. B) Sensibility of DF-1P cells to IFN. DF-1 and DF-1P cells were untreated (-) or treated (+) with chicken IFN alpha (chIFNα) and infected with Vesicular Stomatitis Virus (VSV) expressing GFP virus. DF-1P were unresponsive to chIFNα due to the lack of functional IFNAR2 receptor. Scale bars, 40 µm.
For many years, our research has focused on the molecular characterization of toroviruses, emergent viruses within the Nidovirales order that cause enteric diseases in various species of domestic animals and may pose a zoonotic threat. Building on this expertise, we initiated new projects aimed at developing strategies to control SARS-CoV-2, the virus responsible for the COVID-19 pandemic. Specifically, in collaboration with Dr. Martínez-Sobrido´s group (Texas Biomedical Research Institute, San Antonio, Texas, USA), Dr. Almazán successfully developed an infectious cDNA clone of the SARS-CoV-2 (USA-WA1/2020 strain) using a bacterial artificial chromosome (BAC). This reverse genetics tool is invaluable for exploring key questions in SARS-CoV-2 biology, identifying antiviral agents, and advancing vaccine development against the virus. Our research has also uncovered that SARS-CoV-2 encodes small RNAs that suppress the host’s SERINC5 expression, thereby facilitating viral replication. Additionally, we found that SARS-CoV-2 downregulates several enzymes in the Krebs cycle, adversely affecting mitochondrial function. These findings suggest that therapies aimed at preserving mitochondrial function could be effective in combating SARS-CoV-2 infection.
Scientific Publications
Group Members
Group Leaders
M. Dolores Rodríguez Aguirre
Fernando Almazán Toral
Postdoctoral Researcher
Elisabet Díaz Beneitez
PhD candidates
Altea Martín Martínez
Lab assistant
Antonio Varas

Funding
Our research is funded by national and international institutions as indicated below. For more details, please check the general Funding Section at the CNB website.
News
Real-space heterogeneous reconstruction, refinement, and disentanglement of CryoEM conformational states with HetSIREN
Nat Commun. 2025 Apr 22;16(1):3751. Herreros D, Mata CP, Noddings C, Irene D, Krieger J, Agard DA, Tsai MD, Sorzano COS, Carazo JM. Abstract Single-particle analysis by Cryo-electron microscopy (CryoEM) provides direct access to the conformations of...
Ceftazidime-avibactam use selects multidrug-resistance and prevents designing collateral sensitivity-based therapies against Pseudomonas aeruginosa
Nat Commun. 2025 Apr 9;16(1):3323. Hernando-Amado S, Gomis-Font MA, Valverde JR, Oliver A, Martínez JL Abstract Ceftazidime-avibactam is a β-lactam/β-lactamase inhibitor combination restricted for the treatment of multidrug-resistant infections of Pseudomonas...
El Centro Nacional de Biotecnología estrena nueva imagen para reflejar una etapa de renovación y evolución
El nuevo logotipo está compuesto por una espiral que representa una hélice de ADN vista desde arriba, inspirada en la emblemática “Fotografía 51” de Rosalind Franklin, clave para desentrañar su estructura La presentación de esta imagen coincide con el Día del ADN, una...




