Ultrastructure of Viruses Molecular Aggregates
RESEARCH GROUPS

Jaime Martín-Benito Romero
Group Leader
Research Summary
Our laboratory investigates the structural biology of viral ribonucleoproteins (RNPs) and membrane proteins. We focus on elucidating the architecture and dynamics of RNPs in enveloped single-stranded RNA viruses, including influenza A, using advanced imaging and biophysical techniques. Additionally, we develop systems to determine the structures of membrane proteins, examining their interactions and mechanisms of action. Our aim is to deepen the understanding of viral and membrane protein structures to inform therapeutic development and enhance virological research.
Research Lines
The primary focus of our research group is the study of viral ribonucleoproteins (RNPs) that constitute the virus nucleocapsid in certain enveloped single-stranded RNA viruses. RNPs are macromolecular complexes comprising the genomic RNA, which is bound to multiple monomers of a nucleoprotein, and in some cases, a single copy of the viral polymerase.
Our laboratory has determined the structure of influenza A RNPs at medium resolution and confirmed the presence of this structure in native virions using cryogenic electron tomography and single-particle analysis. Additionally, we have elucidated the structural basis of the transcription process, specifically how RNP produces messenger RNA in influenza A. We are currently expanding these studies to investigate the dynamics of these processes using high-speed atomic force microscopy (HSAFM) and other biophysical techniques. Recently, we unveiled the conformational dynamics of influenza A recombinant RNPs during RNA synthesis using HSAFM and determined the average RNA synthesis rate within the RNP, along with its dependence on nucleotide concentration and the stability of the nascent RNA secondary structure.
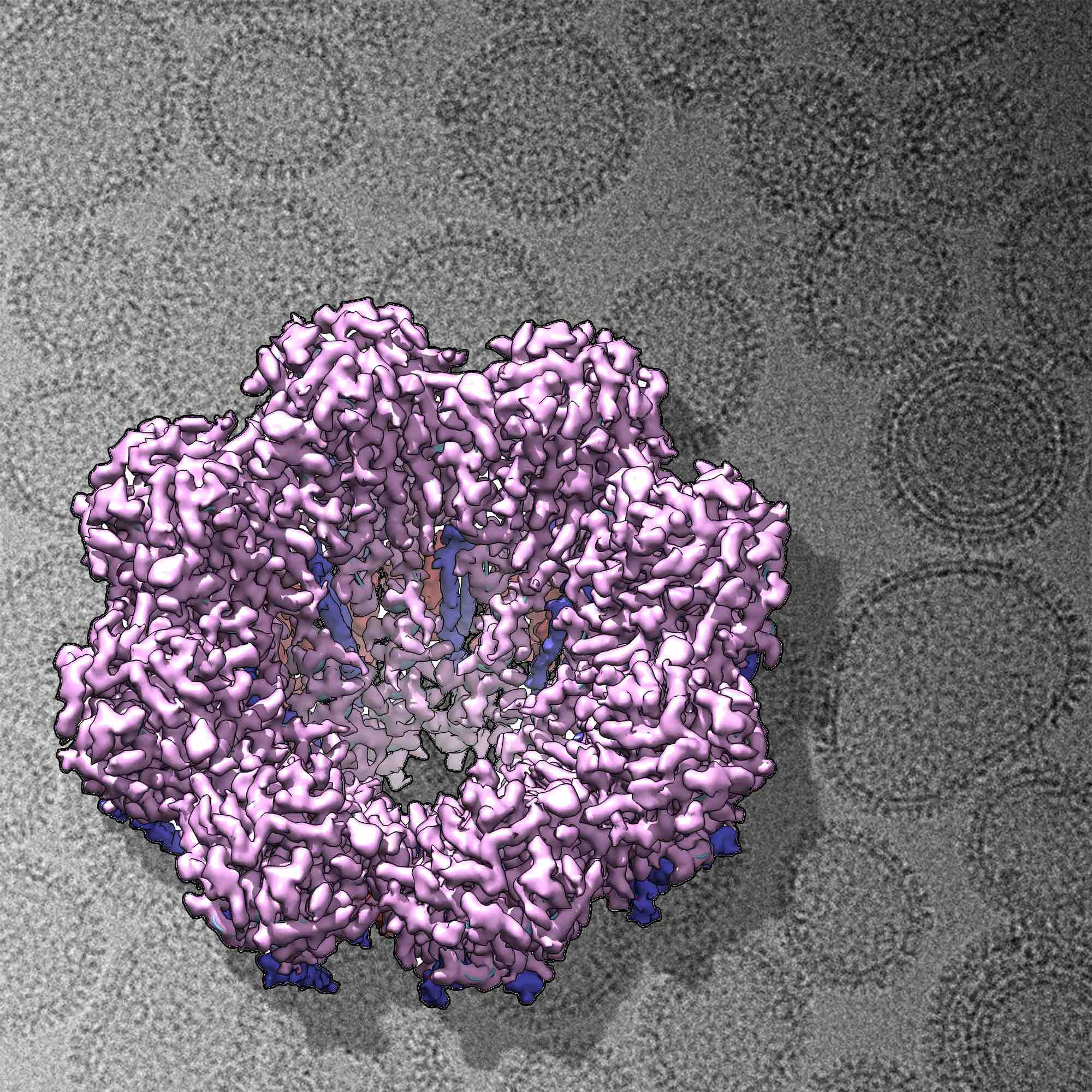
In the context of COVID-19 research, our laboratory has developed a model for structural and biophysical studies on coronaviruses, using the transmissible gastroenteritis virus (TGEV) as a surrogate. We have conducted studies on its biomechanical properties and its resistance to virucidal agents.
Moreover, our laboratory is developing a system to determine the structure of membrane proteins using nanodiscs and liposomes via cryogenic electron microscopy. In this domain, we have resolved the structure of two actinoporins, which are pore-forming proteins, at a resolution of approximately 2 Å. These studies enable us to determine the structure of membrane proteins within the bilayer and to elucidate the interactions between proteins and lipids. Additionally, we have elucidated the pore formation mechanism by determining intermediate structures during the pore-forming process.
Publications
Group Members
Group Leader
Jaime Martín-Benito
Scientific Staff
Diego Carlero Carnero
Rocío Coloma Ciudad
Postdoctoral researcher
María Jesús Rodríguez Espinosa
PhD candidate
Andrea Modrego Guillén
News
Visualizan por primera vez cómo se produce la multiplicación del genoma del virus de la gripe
26 de Julio 2024 Un equipo internacional liderado por el CSIC utiliza Microscopía de fuerza atómica de alta velocidad para ver a escala nanométrica cómo se mueven las moléculas virales para ejercer su acción Estos hallazgos mejoran la comprensión de los procesos...