Viral Molecular Machines
RESEARCH GROUPS

José R. Castón
Group Leader
Research Summary
Our studies address to elucidate structure-function relationships of viral macromolecular complexes, also known as viral nanomachines, which control many fundamental processes in virus life cycle. For that, we have incorporated state-of-the-art approaches to obtaining near-atomic resolution structure directly from electron microscope images.
Research Lines
When viruses are viewed as dynamic containers of an infectious genome, their structural, physical, and biochemical analyses become necessary to understand the molecular mechanisms that control their successful life cycle. Some of these viruses cause serious diseases, and their structural characterization at the highest possible resolution is essential for identifying new targets in the design of future therapies, to inhibit essential functions such as viral assembly and genome replication. In the long-term, the improved knowledge of these viral assemblies can be used to design new virus-based nanostructures for biotechnological applications.
Three-dimensional cryogenic electron microscopy (cryo-EM), which has revolutionized structural biology, is central to determining high-resolution structures of many viral assemblies in near-native conditions. We use Cryo-EM to solve near-atomic structures of infectious virions and other viral complexes with helical or icosahedral symmetry. State-of-the-art approaches now extend beyond purified symmetric capsids and focus on the asymmetric components as the genome and viral polymerases. Asymmetric structures have important functions in many steps of the virus replication cycle, and many of these will be key targets for the development of new antiviral drugs.
Our group studies several viruses with varying levels of complexity, with focus on a number of double-stranded RNA viruses such as infectious bursal disease virus, the human picobirnavirus, and several fungal viruses (Saccharomyces cerevisiae virus L-A, Penicillium chrysogenum virus, and Yadonushivirus), as well as single-stranded RNA viruses such as human rhinovirus and rabbit hemorrhagic disease virus. Mycoviruses might constitute alternative biocontrol agents for fungal diseases, and also help preserve natural environments and improve agricultural resources. In collaboration with several national and international groups, we extended our studies to other macromolecular assemblies such as a2-macroglobulin, a blood plasma proteinase inhibitor of broad specificity, and encapsulins, bacterial nanocages that naturally confine a functional protein cargo such as an enzyme. Structural analysis is complemented by study of mechanical properties by atomic force microscopy, to examine the relationship between physical properties such as rigidity and mechanical resilience, and virus biological function.
Finally, our research establishes the basis for incorporation of heterologous proteins and/or chemicals into viral capsids, considered as nanocontainers or nanocarriers, of potential use for future biotechnological applications.
Cryo-EM structure of Brevibacterium linens encapsulin (BlEnc) at 2.28 Å resolution. BlEnc has a similar fold to the capsid proteins of the viral lineage HK97.
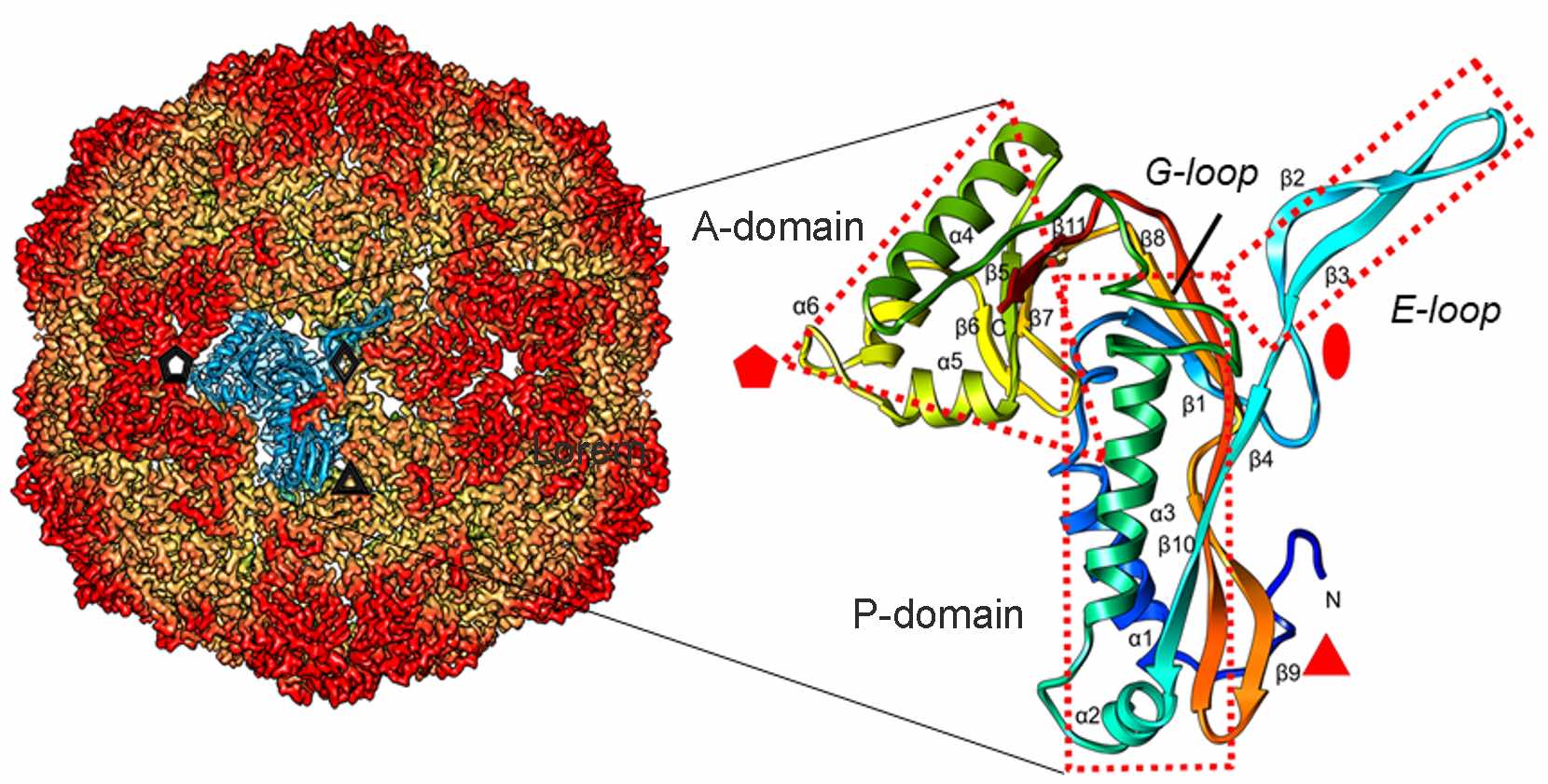
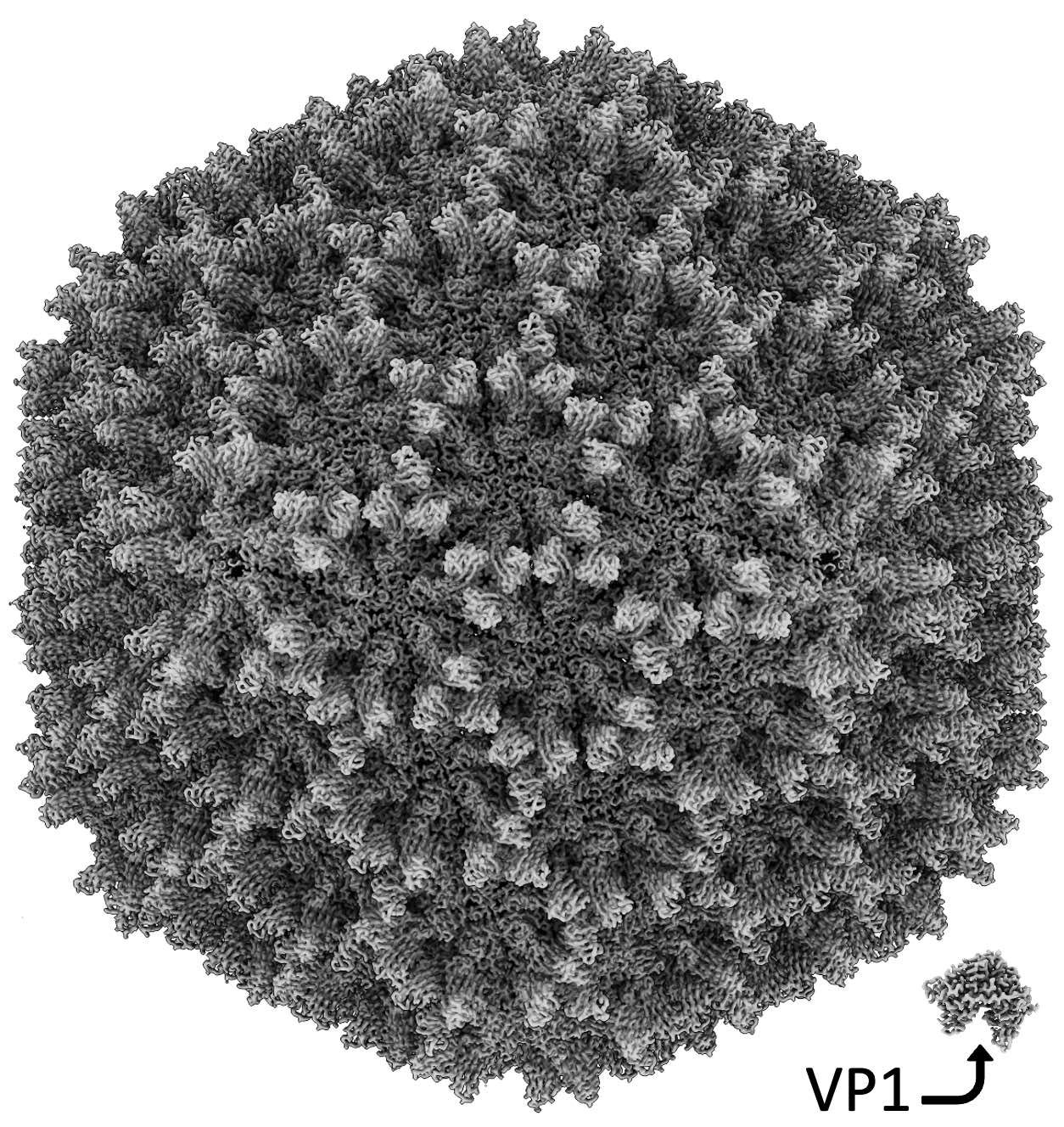
Cryo-EM structure of infectious bursal disease virus (IBDV) at 2.3Å resolution. The ~70-nm-diameter T=13 capsid is built of 780 copies of VP2 (441 residues). Several copies of the viral polymerase VP1 (904 residues, bottom, right corner) are packaged in the capsid interior.
Publications
Group Members
Group Leader
José R. Castón
Postdoctoral Researcher
Ana Ruiz Padilla
PhD candidates
Guy Novoa Benavides
José María Fernández Palacios
Juan Manuel Martínez Romero
Pablo Becana Mínguez



