Nanomedicine, Cancer Immunotherapy and Autoimmune Diseases

Domingo F. Barber
Group Leader
Research Summary
The overall aim of our group is to understand nanomedicine-mediated molecular and cellular mechanisms in novel nanomedicine-based therapeutic applications being developed for the treatment of cancer or autoimmunity, and to use this knowledge to bring these therapies closer to clinical application.
Research Lines
During these years our group has contributed to demonstrate the potential of iron oxide nanoparticles (IONPs) to be used as nanomedicines in different biomedical applications for cancer treatment: targeted drug release, reprogramming of macrophage responses and the tumour microenvironment, induction of intracellular hyperthermia in tumour cells, and magnetic targeting/retention of lymphocytes modified with IONPs in adoptive cell transfer therapies. We have also seen that the accumulation of MNPs in tumour cells induces oxidative stress, as a consequence of IONPs degradation, affecting mitochondrial metabolism. We are therefore investigating whether this effect could be used therapeutically to fight tumours at different levels. We are also expanding the study of the applications of IONPs to other pathologies, and are investigating whether the functionalisation of tolDCs with IONPs, and their subsequent magnetic retention could be used as autoimmune therapy in mouse models of lupus.
The overall aim of our group is to understand nanomedicine-mediated molecular and cellular mechanisms in novel nanomedicine-based therapeutic applications being developed for the treatment of cancer or autoimmunity, and to use this knowledge to bring these therapies closer to clinical application. To fully understand the molecular and cellular mechanisms induced by IONPs at their different levels of action we have several specific objectives:
1) We are investigating how differences in IONPs size, shape, functionalization, or the exposure to an alternating magnetic field (AMF) could further enhance the induction of oxidative stress observed in cells loaded with spherical IONPs, making IONPs with these differences better oxidative stress inducers for oxidative stress-inducing anti-cancer therapy.
2) We are evaluating whether antigen-pulsed IONP-modified dendritic cells (DCs) can activate the anti-tumor immune response. We will differentiate bone marrow-derived DCs (BMDCs) from C57BL/6 mice into mature BMDCs (mDCs) and we will modify them with IONPs for their magnetic targeting to/retention on tumor-draining LN. We will then analyze in vitro the cellular effects of IONPs modification on mDCs using OVA peptide-pulsed mDCs and CD8+ T cells from OT-I mice, and finally, we will evaluate in vivo the magnetic targeting /retention and antitumor efficacy of transferred OVA peptide-pulsed mDCs modified with IONPs.
3) We are evaluating in vivo the magnetic targeting/retention and antitumor efficacy of NK cells modified with IONPs transferred alone, or in combination with CD8+ T cells modified with IONPs or/and IL-15 functionalized IONPs in Adoptive Cell Transfer (ACT) therapies.
4) We are investigating how IONPs contribute to the activation of T cells and NK cells. First, we will analyze how the presence of IONPs on the T cell or NK surface induces to the activation of T cell or NK cell metabolism and whether IONPs could be used to metabolically reprogram T cells or NK cells. Then, we will study the cellular distribution of IONPs during the formation of the immunological synapse (IS), and whether this distribution interferes with the formation of the IS or the activation of T cells or NK cells.
5) We are analyzing whether IONPs-loaded cells eliminate part of their IONPs content by exocytosis of IONPs-containing small extracellular vesicles (sEV), characterize these IONPs-containing sEV, evaluate whether different IONPs coatings could favor IONPs-containing sEV formation, and whether these IONPs-containing sEVcan be magnetically targeted/retained for possible biomedical applications.

Iron oxide nanoparticles impair SARS-CoV-2 infection of cultured cells through two processes induced by the internalization of IONPs: the induction of oxidative stress and changes in the regulation of iron metabolism.
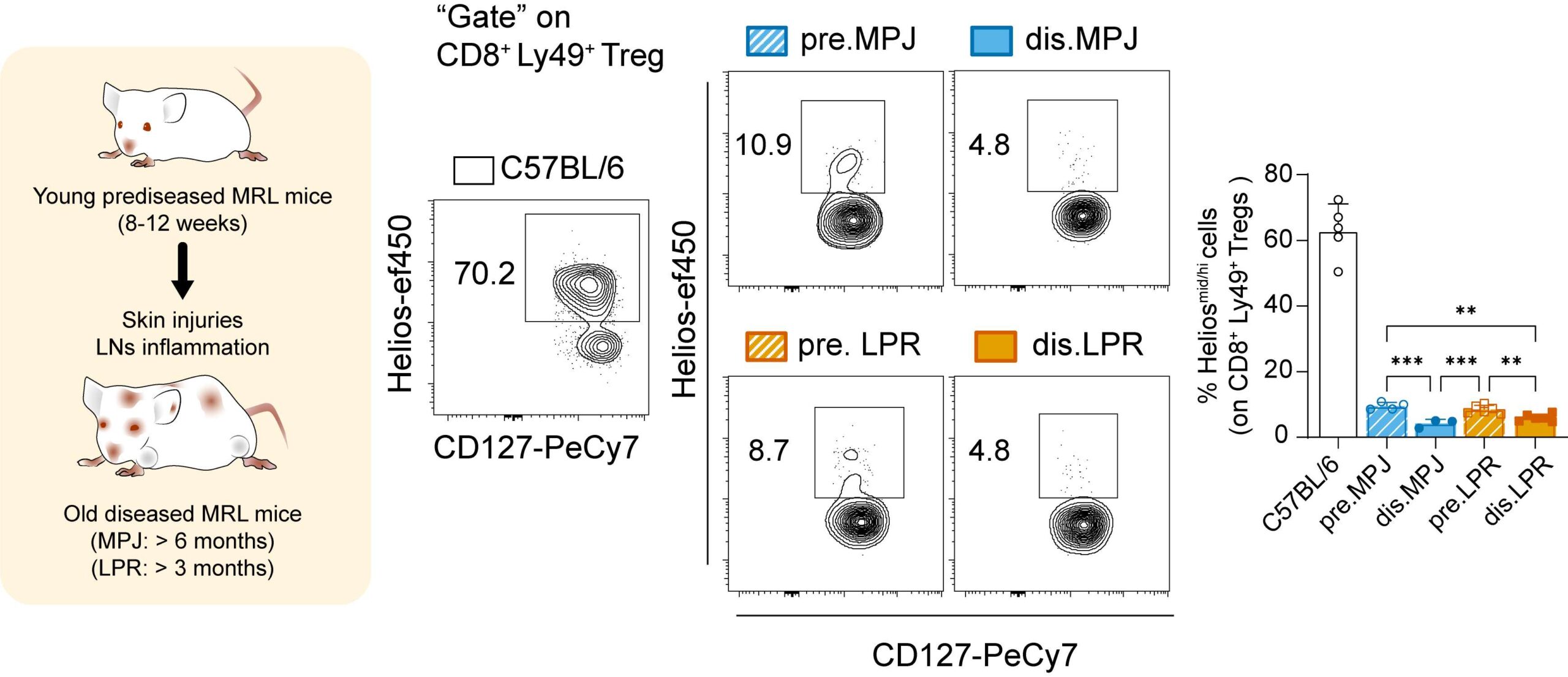
Helios expression on splenic CD8+ Ly49+ Tregs from C57BL/6, MRL/LPR and MRL/MPJ mice.
Publications
Group Members
Group Leader
Domingo F. Barber Castaño
Lab assistant
Sonia Pérez Yagüe
PhD candidates
María Rodríguez Martín
Elena Blanco Arribas

Funding
News
Doctorado en el Grupo Nanomedicina, Inmunoterapia contra el cáncer y enfermedades autoinmunes
IP: Domingo F Barber Buscamos candidatos/as con máster terminado para solicitar ahora un contrato predoctoral de la CAM, y más adelante otros contratos (FPU, Caixa, AECC, etc.) Desarrollaría la tesis doctoral en el estudio de los mecanismos moleculares y celulares...
Identifican un nuevo efecto del uso de nanopartículas de hierro en el crecimiento de células tumorales
Un equipo del CSIC analiza el potencial de distintos recubrimientos de estas nanopartículas como terapias inductoras de estrés oxidativo para tratar al cáncer Las nanopartículas de hierro son una herramienta útil para el transporte dirigido de fármacos antitumorales,...





