Replication, Virus-host Interactions and Protection in Coronavirus
RESEARCH GROUP

Luis Enjuanes
Group Leader

Isabel Sola
Group Leader

Sonia Zuñiga
Group Leader
Research Summary
Our group studies the mechanisms of CoV replication and pathogenesis with a special focus on virus-host interactions in infections with deadly human viruses SARS-CoV-2, SARS-CoV and MERS-CoV. This information is applied to the design of vaccines and the selection of antivirals to protect against acute severe respiratory CoV infections and post-acute sequelae of COVID (PASC). We aim to understand in detail the innate immune response caused by these conditions and develop modulating mechanisms for both young and elderly populations.
Research Lines
Seven coronaviruses (CoV) that infect humans are currently known, including those causing up to 15% of mild respiratory infections. However, three of them, SARS-CoV, MERS-CoV and SARS-CoV-2, represent a human life threat as they can cause severe pneumonia and acute respiratory distress syndrome (ARDS), which are potentially deadly conditions. SARS-CoV-2 is the causative agent of the COVID-19 pandemic.
CoV infections are especially severe in older adults, which respond less efficiently to vaccines. The need for improved preventive measures as well as effective treatments for coronaviruses has increased with the recent pandemic.
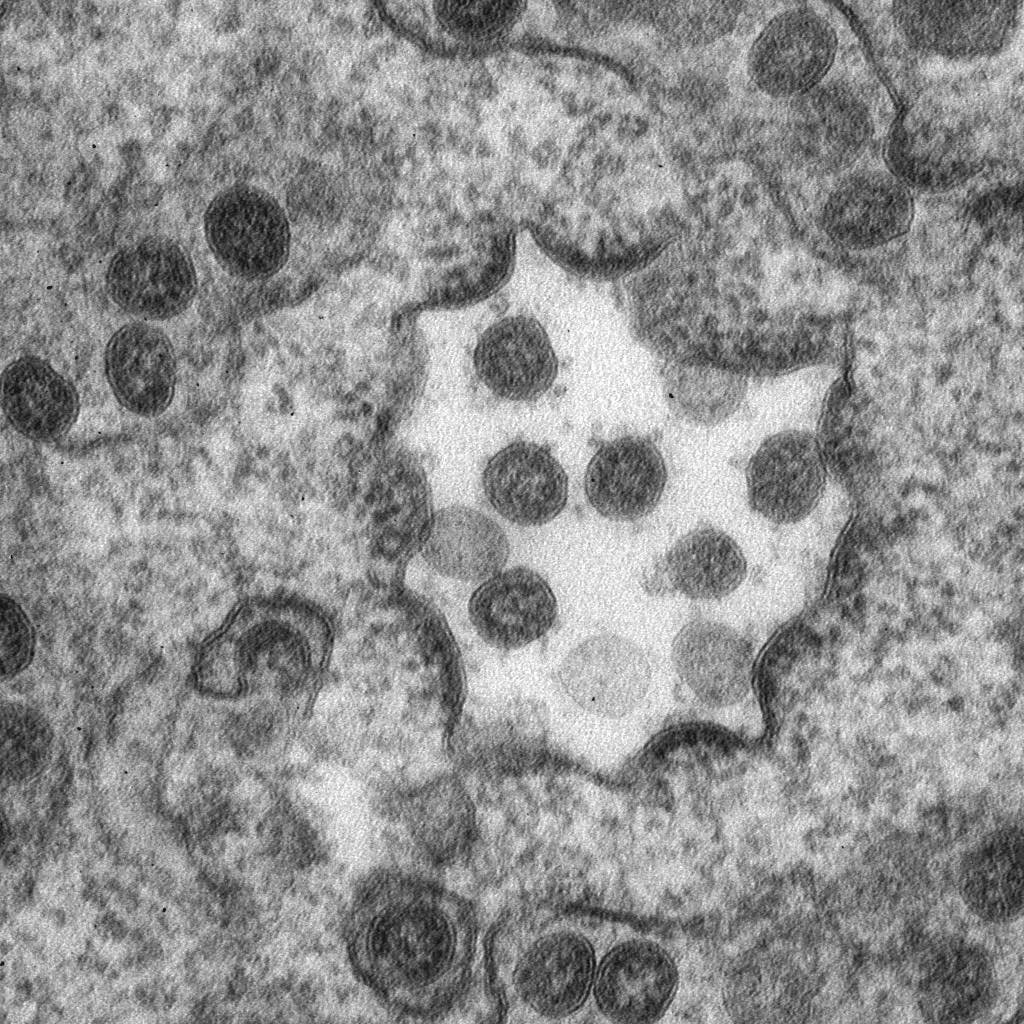
The main aims of our research are:
- To understand the mechanisms of Long-COVID or PASC, two main hypotheses are being investigated. The presence of defective RNA genomes that reduce viral dissemination, inducing proinflammatory reactions in specific tissues, is being studied in human samples and in a mouse model for PASC. Other mechanisms, previously shown by our group to modulate innate responses and inflammation in acute infection are being analyzed. Host factors interacting with virus determinants of virulence that mediate cystic fibrosis transmembrane conductance regulator (CFTR) reduction have being identified as potential targets for antiviral drugs. The contribution of SARS-CoV-2 accessory genes 6, 7a, 7b and 8 is being studied using virus deletion mutants. Post-transcriptional regulation of gene expression mediated either by viral and host small non-coding RNAs or by RNA-protein complexes including viral N and host MOV10 proteins are being analyzed. This information will serve as the basis for new therapeutic interventions.
- Development of next generation SARS-CoV-2 vaccines consisting in replication-competent propagation-deficient RNA replicons and to determine their efficacy in animal model systems. Vaccine development includes: (i) Engineering the SARS-CoV-2 RNA-replicons by deleting or modifying viral genes responsible for propagation and virulence, using reverse genetics; (ii) Development of packaging cell lines that efficiently complement the generation of virus-like particles (VLPs); (iii) Engineering simplified and safer versions by reducing the replicase size; (iv) Optimizing the efficacy of vaccines in older adults by expressing immunomodulatory miRNAs that downregulate immunosenescence.
- To determine the relevance to the inflammatory pathology of post-transcriptional regulation of gene expression. We study the contribution to dysregulated inflammation of small non-coding RNAs (host miRNAs and virus-derived RNAs) and RNA-protein complexes. RNAs and proteins involved in these regulatory networks represent potential antiviral targets.
- Identification of cell-signaling pathways involved in CoV replication and pathology. Understanding how CoV spreads in the body at a molecular level can help select antiviral drugs that inhibit these pathways. We study PBM-PDZ protein-protein interactions and viral accessory genes involved in the innate immune and inflammatory responses, since activation of these pathways is responsible for virulence.

Publications
Group Members
Group Leaders
Luis Enjuanes
Isabel Sola
Sonia Zúñiga
Lab assistants
Jorge Ripoll
Mercedes Ruiz
Lab manager
Margarita González
Scientific Staff
Iván Nombela
Diego Muñoz
Carlos Sánchez
PhD candidates
Jesús Hurtado
Ricardo Requena
María Guzmán
Marta Villarejo
María Rueda
Ana Marchena

Funding
News
Tres investigadores del CNB-CSIC, en la lista de científicos más influyentes del mundo
22 de noviembre 2024 Los investigadores del CNB Luis Enjuanes, José Luis Martínez e Isabel Sola aparecen en la lista Highly Cited Researchers (HCR) del año 2024, elaborada por la plataforma Webofscience Group, de Clarivate Analytics, que reúne a los científicos más...
Luis Enjuanes, nuevo académico de número en la Real Academia de Ciencias
1 de Febrero 2024 Luis Enjuanes, profesor de investigacion del CSIC en el CNB ha ingresado como nuevo académico de número en la Real Academia de Ciencias. Su discurso de ingreso alertó de las consecuencias para la salud de la crisis climática que vivimos: “El cambio...












